Acoustofluidic bioassembly induced morphogenesis for therapeutic tissue fabrication
- PMID: 40324975
- PMCID: PMC12053659
- DOI: 10.1038/s41467-025-59026-4
Acoustofluidic bioassembly induced morphogenesis for therapeutic tissue fabrication
Abstract
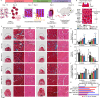
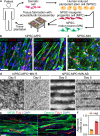
To build in vitro tissues for therapeutic applications, it is essential to replicate the spatial distribution of cells that occurs during morphogenesis in vivo. However, it remains technically challenging to simultaneously regulate the geometric alignment and aggregation of cells during tissue fabrication. Here, we introduce the acoustofluidic bioassembly induced morphogenesis, which is the combination of precise arrangement of cells by the mechanical forces produced by acoustofluidic cues, and the morphological and functional changes of cells in the following in vitro and in vivo cultures. The acoustofluidic bioassembly can be used to create tissues with regulated nano-, micro-, and macro-structures. We demonstrate that the neuromuscular tissue fabricated with the acoustofluidic bioassembly exhibits enhanced contraction dynamics, electrophysiology, and therapeutic efficacy. The potential of the acoustofluidic bioassembly as an in situ application is demonstrated by fabricating artificial tissues at the defect sites of living tissues. The acoustofluidic bioassembly induced morphogenesis can provide a pioneering platform to fabricate tissues for biomedical applications.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.-W.C. is a chief technology officer (CTO) of Cellartgen, Inc., Republic of Korea. The remaining authors declare no competing interests.
Figures






Similar articles
-
Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs.Biofabrication. 2018 Jan 12;10(2):024103. doi: 10.1088/1758-5090/aa9ef1. Biofabrication. 2018. PMID: 29199637
-
Advances and Challenges of Bioassembly Strategies in Neurovascular In Vitro Modeling: An Overview of Current Technologies with a Focus on Three-Dimensional Bioprinting.Int J Mol Sci. 2024 Oct 12;25(20):11000. doi: 10.3390/ijms252011000. Int J Mol Sci. 2024. PMID: 39456783 Free PMC article. Review.
-
Engineering three-dimensional bone macro-tissues by guided fusion of cell spheroids.Front Endocrinol (Lausanne). 2023 Dec 19;14:1308604. doi: 10.3389/fendo.2023.1308604. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38169965 Free PMC article.
-
Acoustofluidic multi-well plates for enrichment of micro/nano particles and cells.Lab Chip. 2020 Sep 21;20(18):3399-3409. doi: 10.1039/d0lc00378f. Epub 2020 Aug 11. Lab Chip. 2020. PMID: 32779677 Free PMC article.
-
Acoustofluidics - changing paradigm in tissue engineering, therapeutics development, and biosensing.Lab Chip. 2023 Mar 1;23(5):1300-1338. doi: 10.1039/d2lc00439a. Lab Chip. 2023. PMID: 36806847 Review.
References
-
- Fatehullah, A., Tan, S. H. & Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol.18, 246–254 (2016). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

