Oral PRI-002 treatment in patients with MCI or mild AD: a randomized, double-blind phase 1b trial
- PMID: 40324978
- PMCID: PMC12053642
- DOI: 10.1038/s41467-025-59295-z
Oral PRI-002 treatment in patients with MCI or mild AD: a randomized, double-blind phase 1b trial
Abstract
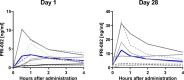
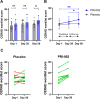
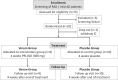
Self-replicating amyloid beta (Aβ) oligomers are considered as one of the major drivers for disrupted synaptic function and plasticity, leading to impaired neuronal viability and progression of Alzheimer's disease (AD). Here, we investigated the safety, tolerability and pharmacokinetics of the anti-oligomeric peptide PRI-002, which was developed to disassemble toxic Aβ oligomers into non-toxic monomers. In a randomized, double-blind, single-center phase 1b trial, 20 patients aged between 50 and 80 years, with mild neurocognitive impairment (MCI) or mild dementia due to AD were recruited. Eligible patients were randomly assigned (1:1) to receive 300 mg PRI-002 once daily (q.d.) or placebo for 28 days. During treatment, study visits were performed on baseline (Day 1), Day 14, Day 28 and an additional follow-up visit on Day 56. Safety assessments were carried out at all visits to determine the primary endpoints. On Day 7 and Day 21 additional phone visits were carried out to assess concomitant meds and AEs. Primary endpoints were nature, frequency, severity, and timing of adverse and serious adverse events (AE/SAEs) and treatment discontinuation. Furthermore, standard laboratory values, electrocardiogram (ECG), electroencephalogram (EEG), magnetic resonance imaging (MRI), and vital signs were assessed. Secondary endpoints included the evaluation of pharmacokinetic characteristics of PRI-002 in plasma and the determination of cerebrospinal fluid (CSF) concentrations of PRI-002. The trial is registered in EudraCT 2020-003416-27 and clinicaltrials.gov NCT04711486 . In the study, 19 out of 20 patients were randomly assigned to PRI-002 (n = 9) or placebo (n = 10) and completed the study. One patient withdrew informed consent before randomization. All primary endpoints were met. Overall, the study drug was well tolerated. In total n = 16 AEs were reported in the verum group, while n = 27 AEs were noted in the placebo group. No SAEs were reported. No significant changes in clinical chemistry, hematology or hematoserology were detected. ECG, EEG and MRI revealed no changes and in detail no ARIA were observed. Pharmacokinetic parameters were unrelated to sex, age, and weight. Furthermore, no significant changes were detected in p-tau, t-tau, Aβ 1-40, Aβ 1-42 and Aβ oligomers in CSF. Patients receiving PRI-002 performed significantly better than those receiving placebo in the CERAD word list at Day 56 (P ≤ 0.05). In conclusion, 28 days of treatment with 300 mg q.d. PRI-002 was well tolerated in patients with MCI or mild dementia due to AD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.W. is cofounder and co-owner of Priavoid GmbH. D.W. is coinventor of PRI-002. O.P. has consulting contracts with Prinnovation GmbH and Priavoid GmbH. This did not have any influence on the interpretation of data. The remaining authors declare no competing interests.
Figures

References
-
- van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med.388, 9–21 (2023). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

