Disulfiram induces redox imbalance and perturbations in central glucose catabolism and metal homeostasis to inhibit the growth of Staphylococcus aureus
- PMID: 40325037
- PMCID: PMC12053631
- DOI: 10.1038/s41598-025-00078-3
Disulfiram induces redox imbalance and perturbations in central glucose catabolism and metal homeostasis to inhibit the growth of Staphylococcus aureus
Abstract
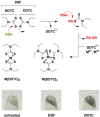
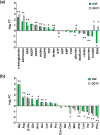
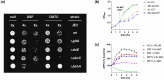
Disulfiram (Antabuse®) is a prescription alcohol sobriety aid that has shown repurposing potential as an antibacterial drug for infections due to Gram-positive bacteria. In this investigation, we sought to define the principal mechanisms that disulfiram operates as a growth inhibitor of Staphylococcus aureus using differential transcriptomic, metabolomic, bioenergetic, and phenotypic growth analyses. The RNA-seq transcriptome analysis revealed that disulfiram induces oxidative stress, redox imbalance, metal acquisition, and the biosynthesis of pantothenate, coenzyme A, thiamine, menaquinone, siderophores/metallophores, and bacillithiol. The metabolomic analysis indicated that disulfiram depletes coenzyme A and attenuates the catabolism of glucose, pyruvate, and NADH. Conversely, disulfiram appeared to up-regulate arginine catabolism for ATP production and accelerate citrate consumption that was attributed to induction of siderophore biosynthesis (i.e., staphyloferrin). The bioenergetic studies further revealed that the primary metabolite of disulfiram (i.e., diethyldithiocarbamate) is likely involved in the mechanism of action as an inhibitor of oxidative phosphorylation and chelating agent of iron and other metals. In the final analysis, disulfiram inhibits the growth of S. aureus by inducing perturbations in central glucose catabolism and redox imbalance (e.g., oxidative stress). Moreover, the chelation of metal ions and antagonism of the respiratory chain by diethyldithiocarbamate are believed to contribute to the inhibition of cell replication.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Hu, P., Jin, L. & Baillie, T. A. Studies on the metabolic activation of disulfiram in rat. Evidence for electrophilic S-oxygenated metabolites as inhibitors of aldehyde dehydrogenase and precursors of urinary N-acetylcysteine conjugates. J. Pharmacol. Exp. Ther.281 (2), 611–617 (1997). PMID: 9152363. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

