Elicitation of liver-stage immunity by nanoparticle immunogens displaying P. falciparum CSP-derived antigens
- PMID: 40325041
- PMCID: PMC12053698
- DOI: 10.1038/s41541-025-01140-x
Elicitation of liver-stage immunity by nanoparticle immunogens displaying P. falciparum CSP-derived antigens
Abstract
A vaccine that provides robust, durable protection against malaria remains a global health priority. Although a breakthrough in the fight against malaria has recently been achieved by the licensure of two vaccines based on the circumsporozoite protein (CSP), the effectiveness and durability of protection can still be improved. Both vaccines contain a portion of CSP that does not include epitopes targeted by recently identified, potently protective monoclonal antibodies, suggesting that newer immunogens can expand the breadth of immunity and potentially increase protection. Here we explored >100 alternative CSP-based immunogens and evaluated the immunogenicity and protection of a large number of candidates, comparing several to the licensed R21 vaccine. The data highlight several general features that improve the stability and immunogenicity of CSP-based vaccines, such as inclusion of the C-terminal domain and high-density display on protein nanoparticle scaffolds. We also identify antigen design strategies that do not warrant further exploration, such as synthetic repeat regions that include non-native repeat cadences. The benchmark R21 vaccine outperformed our best immunogen for immunogenicity and protection. Overall, our data provide valuable insights on the inclusion of junctional region epitopes that will guide the development of potent and durable vaccines against malaria.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


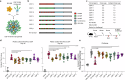
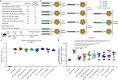

References
-
- WHO. World Malaria Report 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria... (2023).
Grants and funding
LinkOut - more resources
Full Text Sources

