Nasopharyngeal microbiota is influenced by agricultural air pollution in individuals with and without COPD
- PMID: 40325057
- PMCID: PMC12053623
- DOI: 10.1038/s41598-025-00242-9
Nasopharyngeal microbiota is influenced by agricultural air pollution in individuals with and without COPD
Abstract
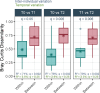
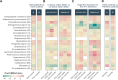
Respiratory health in chronic obstructive pulmonary disease (COPD) is influenced by environmental factors such as air pollution, yet the role of the airway microbiota in this relationship remains unclear. We investigated the association between exposure to air pollution from livestock farms and the nasopharyngeal microbiota in individuals with COPD compared to healthy control subjects. The study included nasopharyngeal swabs from 186 currently non-smoking participants in the Netherlands, including 65 individuals with COPD and 121 without from a regional rural cohort. Additionally, 116 individuals from a population-wide cohort were included as national controls. Samples were taken at three time points over 12 weeks. The nasopharyngeal microbiota was studied using 16 S rRNA gene-based sequencing for all baseline samples and a random selection of 6-weeks and 12-weeks samples. Dispersion models were used to determine the average concentrations of livestock-related PM10, endotoxin, and ammonia at the participants' home addresses. Individuals with COPD had a higher absolute abundance of anaerobic bacteria, such as Peptoniphilus, Anaerococcus, Finegoldia magna, and Prevotella. Importantly, residential exposure to ammonia was identified as the most important driver of the microbial community composition, explaining 6.6% of the variation in nasopharyngeal microbiota in individuals with COPD. Higher ammonia concentrations were associated with decreased levels of key commensals and increased abundance of anaerobic bacteria. Furthermore, individuals living in areas with high livestock density exhibited greater microbial diversity compared to the broader national population. The study highlights the influence of residential exposure to livestock-related air pollution, particularly ammonia, on nasopharyngeal microbiota composition in individuals with COPD. Our findings suggest that environmental factors significantly impact microbial communities and underscore the potential role of anaerobic bacteria in COPD pathology. Future research should further investigate the mechanisms by which environmental air pollutants affect microbial communities and explore potential interventions to mitigate their effects on respiratory health.
Keywords: 16S rRNA amplicon sequencing; Ammonia exposure; Anaerobic bacteria; COPD; Livestock-related air pollution; Nasopharyngeal microbiota.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethical Committee of the University Medical Centre Utrecht (protocol no. 13/533). All participants provided informed consent. Consent to publish: Not Applicable.
Figures

References
-
- National Institute for Public Health and the Environment (RIVM). COPD. (2017). https://www.volksgezondheidenzorg.info/onderwerp/copd
-
- Global National deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet Respir Med.5, 691–706. 10.1016/s2213-2600(17)30293-x (2017). - PMC - PubMed
-
- Hurst, J. R. et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur. J. Intern. Med.73, 1–6. 10.1016/j.ejim.2019.12.014 (2020). - PubMed
-
- Guillien, A. et al. Prevalence and risk factors for COPD in farmers: A cross-sectional controlled study. Eur. Respir J.47, 95–103. 10.1183/13993003.00153-2015 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

