Antibacterial impact of biosynthesized zinc oxide nanoparticles on uropathogenic Escherichia coli and in vivo assessment of physiological and histological alterations
- PMID: 40325065
- PMCID: PMC12053763
- DOI: 10.1038/s41598-025-98060-6
Antibacterial impact of biosynthesized zinc oxide nanoparticles on uropathogenic Escherichia coli and in vivo assessment of physiological and histological alterations
Abstract
Zinc oxide nanoparticles (ZnO NPs) possess various medical potentials that qualify them to be promising antibacterial agents, particularly for uropathogens. The present study investigated in vitro and in vivo antibacterial impact of biosynthesized ZnO NPs against uropathogenic E. coli strain. Values of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of ZnO NPs were detected to be 3.2 mg/mL and 3.9 mg/mL, respectively. The in vivo study included twenty-four female albino rats that were divided into four equal groups: group 1 (control), group 2 (infected), group 3 (infected + ZnO NPs), and group 4 (ZnO NPs). The bactericidal efficacy of ZnO NPs (50 mg/Kg) was confirmed by a recovery percentage of 83.3% after the fourth dose and a survival rate of 100% after eight doses. Erythrocytosis and thrombocytopenia were observed in the infected group, while ZnO NPs-administrated groups exhibited normal red blood cells and platelets counts, and a significant increase in white blood cells count. A significant decrease in urea level and a slight increase in liver enzymes were observed in the infected group, unlike ZnO NPs-administrated groups. Moreover, ZnO NPs-administrated groups exhibited a significant decrease in uric acid and glucose levels. The histological sections of vital body organs showed the aggressive bacterial-induced inflammatory response in stomach, liver, spleen, kidney, and heart of the infected group, whereas ZnO NPs-treated group exhibited effective suppression of the bacterial infection.
© 2025. The Author(s).
Conflict of interest statement
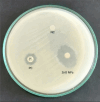
Declarations. Competing interests: The authors declare no competing interests. Dual Publication: Figure 1A, exhibiting Escherichia coli OR064346 strain appearance on CLED agar, and Fig. 2, exhibiting characterization of the biosynthesized ZnO NPs, are reproduced from our previous publications (References 2 and 33, respectively).
Figures








References
-
- Abu-Gharbia, M. A., Al-Arabi, G. & Salem, J. M. Community-acquired urinary tract infections: Epidemiology, etiology, and β-lactam resistance. Sohag J. Sci.9, 47–55. 10.21608/sjsci.2023.226098.1103 (2024). - DOI
-
- Agarwal, H., Kumar, S. V. & Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles: An eco-friendly approach. Res. Eff. Technol.3, 406–413. 10.1016/j.reffit.2017.03.002 (2017). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

