USP18 attenuates endoplasmic reticulum stress via the PERK-eIF2α-ATF4 axis to reduce apoptosis in hepatocellular carcinoma cells
- PMID: 40325079
- PMCID: PMC12052849
- DOI: 10.1038/s41598-025-00540-2
USP18 attenuates endoplasmic reticulum stress via the PERK-eIF2α-ATF4 axis to reduce apoptosis in hepatocellular carcinoma cells
Abstract
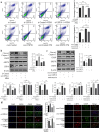
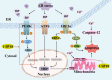
Ubiquitin-specific peptidase 18 (USP18) is a specific interferon-stimulated gene 15 demodifying enzyme that plays an important role in apoptosis. In this study, we investigated the role of USP18 in apoptosis in hepatocellular carcinoma cells, especially its ability to regulate apoptosis through endoplasmic reticulum (ER) stress. We found that protein levels of Bcl-2-associated protein x and cytochrome c were down-regulated by USP18, which suppressed the classical mitochondrial-mediated apoptosis pathway. USP18 also inhibited apoptosis through the unfolded protein response (UPR) pathway by inhibiting the phosphorylation of protein kinase RNA-like endoplasmic reticulum kinase (PERK) and the expression of CCAAT/enhance-binding protein homologous protein, which is a downstream marker molecule of ER stress. The UPR triggered by ER stress eventually led to the cleavage of downstream effecter proteases, including caspase-3, leading to apoptosis. Furthermore, USP18 combined with a PERK agonist regulated apoptosis through the PERK-eukaryotic initiation factor-2α-activating transcription factor 4 axis of the UPR. Our results show that USP18 participates in the regulation of hepatocellular carcinoma cell apoptosis through different pathways, especially the ER stress pathway, and that it plays a complex role in cell stress responses and apoptosis regulation.
Keywords: Apoptosis; Endoplasmic reticulum stress; Hepatocellular carcinoma; Protein kinase RNA-like Endoplasmic reticulum kinase; Ubiquitin-specific peptidase 18.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Melatonin Induces PERK-ATF4 Unfolded Protein Response and Apoptosis in Human Choriocarcinoma Cells.J Pineal Res. 2025 Sep;77(5):e70072. doi: 10.1111/jpi.70072. J Pineal Res. 2025. PMID: 40873119 Free PMC article.
-
Protein-rich foods, sea foods, and gut microbiota amplify immune responses in chronic diseases and cancers - Targeting PERK as a novel therapeutic strategy for chronic inflammatory diseases, neurodegenerative disorders, and cancer.Pharmacol Ther. 2024 Mar;255:108604. doi: 10.1016/j.pharmthera.2024.108604. Epub 2024 Feb 13. Pharmacol Ther. 2024. PMID: 38360205 Free PMC article. Review.
-
[Cannabidiol inhibits neuronal endoplasmic reticulum stress and apoptosis in rats with multiple concussions by regulating the PERK-eIF2α-ATF4-CHOP pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2025 Jun 20;45(6):1240-1250. doi: 10.12122/j.issn.1673-4254.2025.06.13. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40579137 Free PMC article. Chinese.
-
Activation of PERK/eIF2α/ATF4 signaling inhibits ERα expression in breast cancer.Neoplasia. 2025 Jul;65:101165. doi: 10.1016/j.neo.2025.101165. Epub 2025 Apr 18. Neoplasia. 2025. PMID: 40252311 Free PMC article.
-
Glucose sensing and the unfolded protein response.FEBS J. 2025 Jul;292(14):3581-3595. doi: 10.1111/febs.70113. Epub 2025 Apr 24. FEBS J. 2025. PMID: 40272086 Review.
References
-
- Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin.71(3), 209–249 (2021). - PubMed
MeSH terms
Substances
Grants and funding
- 2022QNXM060/the Chongqing Medical Scientific Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau)
- cstc2021jcyj-msxmX0390/the Natural Science Foundation of Chongqing, China
- Chongqing Municipal Finance Bureau, Chongqing Municipal Civil Affairs Bureau [2024] No.21/the National Traditional Chinese Medicine Advantage Specialty
LinkOut - more resources
Full Text Sources
Medical
Research Materials

