Gold Acyclic Diaminocarbene Complexes as Selective and Potent Agents for Multitarget Cancer Therapy
- PMID: 40325346
- PMCID: PMC12093293
- DOI: 10.1021/acs.inorgchem.5c00579
Gold Acyclic Diaminocarbene Complexes as Selective and Potent Agents for Multitarget Cancer Therapy
Abstract
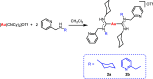
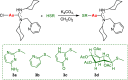
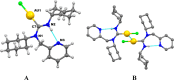
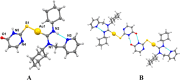
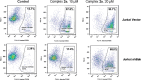
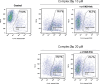
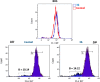
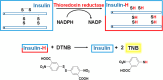
Gold acyclic diaminocarbene (ADC) complexes represent a promising, yet underexplored, class of chemotherapeutics. ADCs offer superior flexibility and stronger sigma donation compared with traditional N-heterocyclic carbenes, making them ideal ligands for stable gold-based drugs. In this study, a series of gold ADC complexes were synthesized via the nucleophilic addition of amines to [AuCl(CNCy)], yielding three structural families: gold-chloride-ADC (chiral and achiral), bis(carbene), and thiolate-gold-ADC complexes. Extensive characterization, including X-ray diffraction, revealed noncovalent interactions, such as hydrogen bonding and aurophilic contacts, that significantly shape their molecular architecture. These complexes exhibit potent cytotoxicity (IC50 in submicromolar) against drug-resistant cancer cell lines (A549, HCT116 WT, Jurkat, MiaPaca2), with some showing high selectivity toward cancer cells over healthy lymphocytes (selectivity index up to 74). Mechanistic investigations indicate that they disrupt mitochondrial function, elevate reactive oxygen species (ROS), and, in the case of bis(carbene) species, bind DNA. Apoptosis is induced at low concentrations, while higher doses trigger alternative death pathways. Notably, they also strongly inhibit thioredoxin reductase (TrxR), comparable in potency to auranofin. The combination of ROS induction, DNA interaction, mitochondrial disruption, and TrxR inhibition highlights the multitargeted anticancer potential of gold-ADC complexes and supports their further development as selective and effective chemotherapeutic agents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Mitochondria-mediated anti-proliferation of triple-negative breast cancer cells by Pd(II)-, Pt(II)-, and Au(III)-NHC complexes of NCN pincers.Dalton Trans. 2025 Jun 24;54(25):10003-10021. doi: 10.1039/d5dt00471c. Dalton Trans. 2025. PMID: 40474752
-
N-heterocyclic carbene gold(I) derivatives with long aliphatic side chains as potential anticancer agents in colon cancer.J Inorg Biochem. 2025 Nov;272:112987. doi: 10.1016/j.jinorgbio.2025.112987. Epub 2025 Jul 7. J Inorg Biochem. 2025. PMID: 40639134
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Gold(I) complexes with NHC ligands functionalized with sulfoxide groups: Design, synthesis, in vitro studies and insights into the mechanism of action as anticancer drugs.J Inorg Biochem. 2025 Oct;271:112957. doi: 10.1016/j.jinorgbio.2025.112957. Epub 2025 May 17. J Inorg Biochem. 2025. PMID: 40440813
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Carver P. L.Metals in Medicine: The Therapeutic Use of Metal Ions in the Clinic. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Carver P. L., Ed.; De Gruyter: Berlin, Boston, 2019; pp 1–16.10.1515/9783110527872-001. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

