Nomogram-based prediction of the prognosis in patients with free floating venous thrombus after closed traumatic fracture
- PMID: 40325423
- PMCID: PMC12054307
- DOI: 10.1186/s12880-025-01695-0
Nomogram-based prediction of the prognosis in patients with free floating venous thrombus after closed traumatic fracture
Abstract
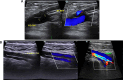
Background: Free-floating venous thrombosis (FFVT), a distinct subtype of deep vein thrombosis (DVT), is associated with pulmonary thromboembolism (PTE) and carries a high mortality risk.
Objective: This study aimed to develop a nomogram to predict the prognosis of FFVT in patients with closed traumatic fractures.
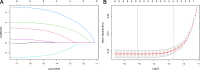
Materials and methods: A retrospective analysis of clinical and ultrasound data from 326 patients with FFVT post-closed traumatic fractures was conducted. Patients were divided into training (n = 240, January 2019-June 2023) and validation (n = 86, June 2023-June 2024) sets. Prognostic risk factors were identified using LASSO and multivariable logistic regression. A nomogram was constructed using R Studio, and its predictive accuracy was validated via calibration curves, receiver operating characteristic (ROC) analysis, and external validation.
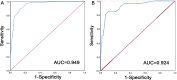
Results: Independent risk factors for FFVT progression to closed thrombus included D-dimer levels, FFVT location, collateral blood flow volume around the thrombus, and thrombus margins (P < 0.05). The model demonstrated high discriminative ability, with a C-index of 0.945. ROC analysis revealed areas under the curve (AUC) of 0.949 (training set) and 0.924 (validation set). Calibration curves confirmed strong agreement between predicted and observed outcomes.
Conclusion: The nomogram provides an accurate prognostic tool for FFVT in patients with closed traumatic fractures, aiding clinical decision-making to improve patient outcomes.
Clinical trial number: Not applicable.
Keywords: Free floating venous thrombosis; Nomogram; Prediction model; Prognosis; Risk factors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the Medical Ethics Committee of Tianjin Hospital (2024MER204). Written informed consent was waived due to the nature of retrospective study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Cancer-Perez S, Alfayate-García J, Vicente-Jiménez S, Ruiz-Muñoz M, Dhimes-Tejada FP, Gutiérrez-Baz M, et al. Symptomatic common carotid Free-Floating Thrombus in a Covid-19 patient, case report and literature review. Ann Vasc Surg. 2021;73:122–8. 10.1016/j.avsg.2021.02.008. Epub 2021/03/11. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

