Modulation of senescent Lepr+ skeletal stem cells via suppression of leptin-induced STAT3‒FGF7 axis activation alleviates abnormal subchondral bone remodeling and osteoarthritis progression
- PMID: 40325465
- PMCID: PMC12054238
- DOI: 10.1186/s13287-025-04342-1
Modulation of senescent Lepr+ skeletal stem cells via suppression of leptin-induced STAT3‒FGF7 axis activation alleviates abnormal subchondral bone remodeling and osteoarthritis progression
Abstract
Background: Recent studies have suggested that targeting senescent cells in joint tissues may alleviate osteoarthritis (OA) progression. However, this strategy encounters significant challenges, partially due to the high degree of cellular heterogeneity in osteoarthritic tissues. Moreover, little information is available on the role of skeletal stem cell (SSC) senescence, as compared to differentiated cells, in OA progression.
Methods: In this study, single-cell RNA sequencing (scRNA-seq) on articular cartilages and subchondral bones of the knee joints of mice with post-traumatic osteoarthritis (PTOA) were performed. Further in vivo and in vitro studies were performed to reveal the role and mechanisims of senescent SSCs during the development of OA lesions and progression by microCT, pathological analysis, and functional gain and loss experiments. The one-way ANOVA was used in multiple group data analysis.
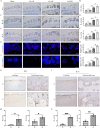
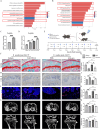
Results: scRNA-seq and pathological data demonstrated that the leptin receptors (Lepr) positive SSCs underwent cellular senescence during OA progression. In addition, the leptin-Lepr signaling pathway induced signal transducer and activator of transcription 3 (STAT3) expression in SSCs, which consequently augmented the transcription of fibroblast growth factor 7 (FGF7). Further scRNA-seq and in vivo analyses revealed that FGF7 exacerbated abnormal bone remodeling in subchondral bones and OA progression by enhancing bone formation and suppressing bone resorption. In vitro analysis revealed that FGF7 induced the osteogenic differentiation of SSCs but inhibited osteoclastogenesis in a concentration-dependent manner.
Conclusions: In summary, our findings demonstrate that the leptin-Lepr signaling pathway promotes SSC senescence and exacerbates subchondral bone remodeling by activating the STAT3-FGF7 axis during OA progression, which may shed light on novel therapeutic strategies for OA.
Keywords: Heterogeneity of Cellular senescence; Leptin-Lepr signaling; Osteoarthritis; STAT3-FGF7 axis; Single-cell sequencing; Skeletal stem cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal research involved in this work was approved by the Institutional Animal Care and Use Committee of Military Medical Sciences. Title of the approved project: National Science Foundation for “The regulatory effects of chondrogenic progenitor cells on the osteoclast formation and bone remodeling in osteoarthritis”. The initial Ethics approval (Approval No: IACUC-DWZX-2024-P523) was obtained on February 27, 2024. The care and use of animals were performed strictly following the regulations on the management of experimental animals. The research on human tibia plateau specimens samples involved in this work was approved by the Institutional Review Board at the Chinese People’s Liberation Army General Hospital Title of the approved project: “discarded biological material from knee osteoarthritis patients with total knee arthroplasty for scientific research projects”, The initial Ethics approval (2018-03-15) was obtained on March 15, 2018. Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Frydendal T, Christensen R, Mechlenburg I, Mikkelsen LR, Varnum C, Graversen AE, Kjaersgaard-Andersen P, Revald PH, Hofbauer C, Bieder MJ, et al. Total hip replacement or resistance training for severe hip osteoarthritis. N Engl J Med. 2024;391:1610–20. 10.1056/NEJMoa2400141. - PubMed
-
- Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384:51–9. 10.1056/NEJMcp1903768. - PubMed
-
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9. - PubMed
-
- Jing Y, Jiang X, Ji Q, Wu Z, Wang W, Liu Z, Guillen-Garcia P, Esteban CR, Reddy P, Horvath S, et al. Genome-wide CRISPR activation screening in senescent cells reveals SOX5 as a driver and therapeutic target of rejuvenation. Cell Stem Cell. 2023;30(1452–1471): e1410. 10.1016/j.stem.2023.09.007. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

