Evolution of treatment practices and outcomes in multiple myeloma during 2013-2022: a Finnish real world registry study
- PMID: 40325790
- PMCID: PMC12067984
- DOI: 10.2340/1651-226X.2025.42647
Evolution of treatment practices and outcomes in multiple myeloma during 2013-2022: a Finnish real world registry study
Abstract
Background and purpose: Multiple myeloma (MM) is a heterogenous hematologic malignancy with an evolving treatment landscape. This Finnish real-world evidence study clarifies the evolution of treatment practices and outcomes over recent years.
Methods: This retrospective analysis included 1,733 patients with MM diagnosed between 2013 and 2022. The cohort was identified and electronic health record data were collected from four hospital data lakes and linked to national registries, covering 54% of Finland's population. Patients were divided by stem cell transplantation (SCT) status into a SCT group (512 patients) and a non-SCT group (1,221 patients), and further by diagnosis period (2013-2017 vs. 2018-2022).
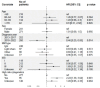
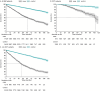
Results: The average age of the patients was 71.3 years at diagnosis. Novel therapeutic use markedly increased during the follow-up, especially lenalidomide as part of frontline and maintenance therapy in SCT patients. For SCT patients the 4-year survival rate improved from 81.7% (95% confidence interval [CI]: 76.4-86.0) in 2013-2017 to 93.0% (95% CI: 87.0-96.3) in 2018-2022. For non-SCT-patients, the median overall survival (OS) increased slightly from 41.3 months (95% CI: 38.1-45.6) in the 2013-2017 period to 43.8 months (95% CI: 39.8-55.3) in the 2018-2022 period, although the difference was not statistically significant. High risk cytogenetics and high International Staging System class appeared to persist as factors indicating shorter OS.
Interpretation: While advancements of novel drugs have resulted in a notable survival benefit for patients undergoing SCT, the survival of non-SCT-patients has remained comparatively static. New approaches in the treatment of MM for elderly and frail non-SCT patients are warranted.
Conflict of interest statement
MW and KN are employees of Johnson & Johnson. AP declares honoraria for lectures from Behring and Abbvie and has participated in the Scientific Advisory Boards Abbvie, Janssen-Cilag, Novartis, Pfizer, Sanofi and Takeda. MP declares honoraria for lectures from and membership on advisory boards with BMS, Janssen, Pfzer and Sanofi, and consultancy for Janssen and Pfzer. JE, JV and RM have no interests to declare.
Figures



References
-
- Cancer Registry, Finland. Syöpärekisteri . Cancer statistics [Internet]. [cited 2023 May 22]. Available from: https://cancerregistry.fi/statistics/cancer-statistics/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

