Hypoxia and Hypoxia-Reoxygenation Potentiate Helicobacter pylori Infection and Gastric Epithelial Cell Proliferation
- PMID: 40325849
- PMCID: PMC12053057
- DOI: 10.1002/cam4.70860
Hypoxia and Hypoxia-Reoxygenation Potentiate Helicobacter pylori Infection and Gastric Epithelial Cell Proliferation
Abstract
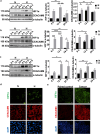
Introduction: The gastric epithelium experiences intermittent hypoxia due to various physiological and pathological conditions. However, the impact of hypoxia and hypoxia-reoxygenation of gastric epithelial cells (GECs) on Helicobacter pylori-mediated gastric cancer (GC) has never been investigated. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) facilitate H. pylori adhesion onto GECs. We evaluated the effect of hypoxia and hypoxia-reoxygenation on CEACAM6-mediated H. pylori binding, infection, reactive oxygen species (ROS) generation, and GEC proliferation.
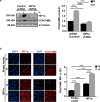
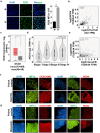
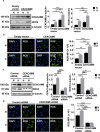
Methods: Hypoxia-inducible factor 1 (HIF1α) and CEACAM6 levels were assessed in various GECs. ROS were measured using 2',7'-dichlorofluorescin diacetate (DCFDA). Bioinformatics analyses were performed to identify the most prominent stomach adenocarcinoma (STAD)-associated NADPH oxidase (NOX) followed by validation by overexpression/suppression studies and western blotting. GC biopsies were examined by immunofluorescence microscopy. Hypoxia-exposed, reoxygenated, or control cells were compared for ROS generation and H. pylori infection. MTT assay determined cell proliferation.
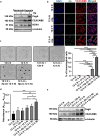
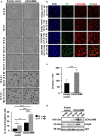
Results and conclusions: Hypoxia and HIF1 mediated upregulation of CEACAM6 in GECs. CEACAM6 significantly promoted ROS generation by inducing NOX4 in hypoxic GECs. HIF1α, CEACAM6, and NOX4 upregulation was detected in gastritis and GC tissues. H. pylori infection significantly increased in hypoxia-exposed GECs as compared to normoxic GECs. Infection of hypoxia-reoxygenated GECs also resulted in significantly increased CEACAM6 and NOX4-mediated ROS generation compared to normoxic GECs. In addition, adhesion of H. pylori, cytotoxin-associated gene A (CagA) translocation, and GEC proliferation were significantly enhanced in hypoxia-reoxygenated GECs. Collectively, this study established that hypoxia and hypoxia-reoxygenation of GECs facilitate H. pylori infection and infection-mediated GEC proliferation.
Keywords: Helicobacter pylori, hypoxia; NADPH oxidase 4; carcinoembryonic antigen; hypoxia‐inducible factor 1; hypoxia‐reoxygenation; reactive oxygen species.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sharma P. K., Suri T. M., Venigalla P. M., et al., “Atrophic Gastritis With High Prevalence of <styled-content style="fixed-case"> Helicobacter pylori </styled-content> Is a Predominant Feature in Patients With Dyspepsia in a High Altitude Area,” Tropical Gastroenterology 35 (2014): 246–251. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

