Real-life experience of luspatercept in transfusion-dependent lower risk myelodysplastic syndrome patients
- PMID: 40325906
- PMCID: PMC12234265
- DOI: 10.1111/bjh.20102
Real-life experience of luspatercept in transfusion-dependent lower risk myelodysplastic syndrome patients
Abstract
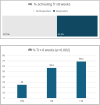
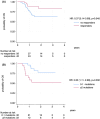
Luspatercept has been approved for the treatment of anaemia in transfusion-dependent (TD) patients with lower risk (LR) myelodysplastic syndromes (MDS) after erythroid-stimulated agent (ESA) failure, according to the results of the MEDALIST trial. In this multicentre retrospective study, we report efficacy and safety data of luspatercept administered in 98 TD LR-MDS patients after ESA failure. The percentage of patients that stopped luspatercept due to adverse events was comparable to that reported in the MEDALIST study. Furthermore, we observed that 44.3% patients who had completed 24 weeks of follow-up achieved transfusion independence lasting longer than 8 weeks, compared to 38% in the MEDALIST trial. These positive results may be attributed to the inclusion of patients with lower transfusion needs in our study. All responses were observed within 8 months since luspatercept onset and many were long-lasting, even in the high-transfusion burden patient group. In addition, response to luspatercept and the presence of less than two mutations independently predicted for longer overall survival. Overall, our results confirm luspatercept's safety and efficacy in TD LR-MDS patients who have experienced ESA failure in a real-world setting.
Keywords: lower risk MDS; luspatercept; response; survival; transfusion dependence.
© 2025 The Author(s). British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures



References
-
- Garcia‐Manero G, Chien KS, Montalban‐Bravo G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95(11):1399–1420. - PubMed
-
- Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Arango Ossa JE, Nannya Y, et al. Molecular International Prognostic Scoring System for myelodysplastic syndromes. NEJM Evid. 2022;1(7):EVIDoa2200008. - PubMed
-
- Fenaux P, Platzbecker U, Ades L. How we manage adults with myelodysplastic syndrome. Br J Haematol. 2020;189(6):1016–1027. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

