Transcutaneous Carbon Dioxide Monitoring During Weaning From Mechanical Ventilation in Children: The WeanCO2 Study
- PMID: 40325912
- PMCID: PMC12053114
- DOI: 10.1002/ppul.71115
Transcutaneous Carbon Dioxide Monitoring During Weaning From Mechanical Ventilation in Children: The WeanCO2 Study
Abstract
Introduction: Spontaneous breathing trial (SBT) is recommended during weaning from mechanical ventilation (MV), but objective and easy tools lack to identify pediatric weaning failure. We aimed to assess whether changes in estimated arterial CO₂ (PaCO₂) derived from transcutaneous measurements (PTCCO₂) were associated with pediatric weaning failure.
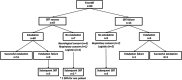
Methods: Children (age 72 h -18 years) with MV > 12 h were continuously monitored using a transcutaneous sensor to estimate PaCO₂ from skin CO₂ tension (PTCCO₂). Values were recorded during SBT (30 min on positive end-expiratory pressure (PEEP) +5 cmH2O, with pressure support of +5 cmH2O for endotracheal tubes with internal diameter ≤ 3.5 mm), then up to 6 h after extubation. Mean PTCCO2 and PTCCO2 changes during SBT, and after extubation, were retrospectively collected to evaluate their association with SBT failure and extubation failure (reintubation within 48 h).
Results: Eighty children (median [IQR] age 1.1 [0.3; 8.7] years) were included, with 89 SBT (14 failures, 75 successes). Sixty-four patients were extubated following their first SBT, with 10 (16%) extubation failures. PTCCO2 changes were not associated with SBT and extubation failures. Patients who failed extubation had a higher mean PTCCO2 value after extubation as compared to those who were successfully extubated (mean PTCCO2 of 51.8 [46.2; 55.4] vs. 42.3 [37.5; 47.2] mmHg, p = 0.02). The difference between the maximal PTCCO2 value within the 2 h following extubation and the value at extubation were higher in patients who failed extubation (ΔPTCCO2 of 20 [9.1; 26] vs. 6.8 [2.9; 9.7] mmHg, p < 10-2).
Conclusion: Early post-extubation increase in estimated PaCO₂ was associated with extubation failure, whereas PTCCO₂ changes during SBT were not.
Keywords: children; extubation; mechanical ventilation; spontaneous breathing trial; transcutaneous carbon dioxide.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
M. Vedrenne‐Cloquet reports a loan of a PTCCO2 monitoring device from Sentec to conduct the study, with no influence on the study protocol or the results. The other authors declare no conflicts of interest.
Figures


References
-
- Abu‐Sultaneh S., Iyer N. P., Fernández A., et al., “Executive Summary: International Clinical Practice Guidelines for Pediatric Ventilator Liberation, a Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Document,” American Journal of Respiratory and Critical Care Medicine 207, no. 1 (January 2023): 17–28. - PMC - PubMed
-
- Amaddeo A. and Fauroux B., “Oxygen and Carbon Dioxide Monitoring During Sleep,” Paediatric Respiratory Reviews 20 (September 2016): 42–44. - PubMed
-
- Bhalla A. K., Khemani R. G., Hotz J. C., Morzov R. P., and Newth C. J., “Accuracy of Transcutaneous Carbon Dioxide Levels in Comparison to Arterial Carbon Dioxide Levels in Critically Ill Children,” Respiratory Care 64, no. 2 (February 2019): 201–208. - PubMed
-
- Henao‐Brasseur J., Bedel J., Mutlu G., et al., “Transcutaneous CO2 Monitoring: A New Tool to Identify Spontaneous Breathing Trial Failure During Weaning From Mechanical Ventilation. A Pilot Cohort Study,” Intensive Care Medicine 42, no. 6 (June 2016): 1078–1079. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

