Sorbent Mediated Electrocatalytic Reduction of Dilute CO2 to Methane
- PMID: 40326475
- PMCID: PMC12082630
- DOI: 10.1021/jacs.4c18303
Sorbent Mediated Electrocatalytic Reduction of Dilute CO2 to Methane
Retraction in
-
Retraction of "Sorbent Mediated Electrocatalytic Reduction of Dilute CO2 to Methane".J Am Chem Soc. 2025 Nov 12;147(45):42152. doi: 10.1021/jacs.5c15044. Epub 2025 Oct 31. J Am Chem Soc. 2025. PMID: 41170842 Free PMC article. No abstract available.
Abstract
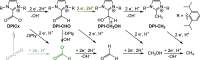
Efficient CO2 utilization is a critical component of closing the anthropogenic carbon cycle. Most studies have focused on the use of pure streams of CO2. However, CO2 is generally available only in dilute streams, which requires capture by sorbents followed by energy-intensive regeneration to release concentrated CO2. Direct utilization of sorbed-CO2 avoids the costly regeneration step, and the sorbent-CO2 interaction can kinetically activate CO2 to tune its reactivity toward products that could otherwise be inaccessible with direct CO2 reduction. We demonstrate that an N-heterocyclic carbene, 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene (DPIy), quantitatively reacts with CO2 from dilute streams (0.04 and 10%) to form the sorbent-CO2 substrate 1,3-bis(2,6-diisopropylphenyl)imidazolium-2-carboxylate (DPICx). Electrocatalyst iron tetraphenylporphyrin chloride (Fe(TPP)Cl) typically reduces CO2 to CO; however, with DPICx as the substrate, the eight-electron reduced product methane (CH4) is produced with a high Faradaic efficiency (>85%) and regeneration of the sorbent DPIy. In addition to the overall energy and capital advantages of integrated CO2 capture and conversion, this result illustrates how sorbents can serve a dual purpose for both CO2 capture and chemical auxiliary purposes to access unique products. CO2 has a spectrum of reactivity with different types of sorbents; thus, these studies demonstrate how sorbent-CO2 interactions can be leveraged for integrated capture and utilization platforms to access a wider range of CO2-derived products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Balamurugan M.; Merakeb L.; Nam K. T.; Robert M.. Electrochemical CO2 Reduction. In Chemical Valorisation of Carbon Dioxide, Stefanidis G.; Stankiewicz A. Eds.; Royal Society of Chemistry, 2022; p 1804.
-
- Deutsch T. G.; Baker S.; Agbo P.; Kauffman D. R.; Vickers J.; Schaidle J. A.. Summary Report of the Reactive CO2 Capture: Process Integration for the New Carbon Economy Workshop, February 18-19, 2020. 2020. https://www.nrel.gov/docs/fy21osti/78466.pdf (accessed 05/01/2024).
-
- Freyman M. C.; Huang Z.; Ravikumar D.; Duoss E. B.; Li Y.; Baker S. E.; Pang S. H.; Schaidle J. A. Reactive CO2 capture: A path forward for process integration in carbon management. Joule 2023, 7 (4), 631–651. 10.1016/j.joule.2023.03.013. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

