A function of Spalt proteins in heterochromatin organization and maintenance of genomic DNA integrity
- PMID: 40326666
- PMCID: PMC12091872
- DOI: 10.1242/dev.204258
A function of Spalt proteins in heterochromatin organization and maintenance of genomic DNA integrity
Abstract
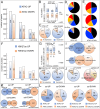
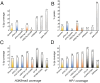
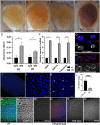
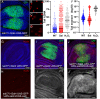
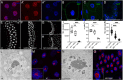
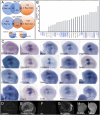
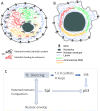
The conserved Spalt proteins regulate gene expression and cell fate choices during multicellular development, generally acting as transcriptional repressors in different gene regulatory networks. In addition to their roles as DNA sequence-specific transcription factors, Spalt proteins show a consistent localization to heterochromatic regions. Vertebrate Spalt-like proteins can act through the nucleosome remodeling and deacetylase complex to promote closing of open chromatin domains, but their activities also rely on interactions with DNA methyltransferases or with the lysine-specific histone demethylase LSD1, suggesting that they participate in multiple regulatory mechanisms. Here, we describe several consequences of loss of Spalt function in Drosophila cells, including changes in chromatin accessibility, generation of DNA damage, alterations in the localization of chromosomes within the nucleus in the salivary glands and misexpression of transposable elements. We suggest that these effects are related to roles of Spalt proteins in the regulation of heterochromatin formation and chromatin organization. We propose that Drosophila Spalt proteins have two complementary functions, acting as sequence-specific transcriptional repressors on specific target genes and regulating more global gene silencing through the generation or maintenance of heterochromatic domains.
Keywords: Drosophila; Gene expression; Heterochromatin; Nuclear lamina; Nucleolus; Spalt proteins.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
References
-
- Ballmer, D., Tardat, M., Ortiz, R., Graff-Meyer, A., Ozonov, E. A., Genoud, C., Peters, A. H. F. M. and Fanourgakis, G. (2023). HP1 proteins regulate nucleolar structure and function by secluding pericentromeric constitutive heterochromatin. Nucleic Acids Res. 51, 117-143. 10.1093/nar/gkac1159 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- PID2022-141894OB-C21/Spanish National Plan for Scientific and Technical Research and Innovation
- PID2022-141894OB-C21/Secretaría de Estado de Investigación, Desarrollo e Innovación
- DeCRyPT (787611)/ERC_/European Research Council/International
- DFG-SPP 2202/Deutsche Forschungsgemeinschaft
- Consejo Superior de Investigaciones Científicas
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

