Enhanced pain relief with guanfacine as an adjuvant for trigeminal nerve blocks: insights from a PheWAS-guided randomized controlled study
- PMID: 40326696
- PMCID: PMC12491861
- DOI: 10.1093/pm/pnaf054
Enhanced pain relief with guanfacine as an adjuvant for trigeminal nerve blocks: insights from a PheWAS-guided randomized controlled study
Abstract
Background: Trigeminal neuralgia and painful trigeminal neuropathy are severely painful conditions which are often difficult to treat with medications. A Phenome-Wide Association Study (PheWAS) analysis identified an association between single-nucleotide variants in the alpha-2 adrenergic receptor (ADRA2) subtype B, a G protein-coupled receptor expressed on peripheral and central presynaptic terminals, and an increased risk for trigeminal nerve disorders. We hypothesized that adding the ADRA2 agonist guanfacine to routine care trigeminal nerve injections would provide enhanced pain relief in trigeminal neuralgia than routine care alone.
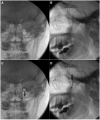
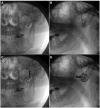
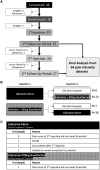
Methods: PheWAS analysis identified the drug target/indication pair. The trial was a single-center, prospective, randomized, controlled, double-blinded, 2-way crossover trial for patients with pain attributed to the trigeminal nerve. Injections of lidocaine alone or lidocaine + 250 µg guanfacine were delivered to the V2 and V3 branches of the trigeminal nerve by experienced clinicians. Patients reported pain using a Numeric Rating Scale (NRS) and quality of life measures (using the PROMIS Global Physical Health, Mental Health, and Pain Interference surveys).
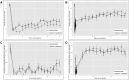
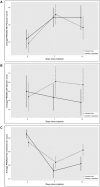
Results: NRS pain scores over the first 8 h post-injection were significantly lower in the interventional group (guanfacine + lidocaine) compared to active control (P < .001), but not from day 1 through day 14. There was no significant difference between treatment groups in time to recovery of baseline pain intensity (primary outcome), PROMIS outcomes, or adverse events (AEs). No serious AEs were reported.
Conclusions: The addition of 250 µg guanfacine to lidocaine for a nerve block procedure enhanced analgesia compared to lidocaine alone within the first 8 h post-injection.
Clinical trial number: NCT03865940, https://clinicaltrials.gov/study/NCT03865940.
Keywords: drug repurposing; guanfacine; nerve block; neuropathic pain; trigeminal neuralgia.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Academy of Pain Medicine.
Figures

References
-
- Bendtsen L, Zakrzewska JM, Heinskou TB, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020;19(9):784-796. - PubMed
-
- Choi YK, Liu J. The use of 5% lidocaine for prolonged analgesia in chronic pain patients: a new technique. Reg Anesth Pain Med. 1998;23(1):96-100. - PubMed
-
- Radwan IA, Saito S, Goto F. High-concentration tetracaine for the management of trigeminal neuralgia: quantitative assessment of sensory function after peripheral nerve block. Clin J Pain. 2001;17(4):323-326. - PubMed
-
- Han KR, Kim C, Chae YJ, Kim DW. Efficacy and safety of high concentration lidocaine for trigeminal nerve block in patients with trigeminal neuralgia. Int J Clin Pract. 2008;62(2):248-254. - PubMed
-
- Perloff MD, Chung JS. Urgent care peripheral nerve blocks for refractory trigeminal neuralgia. Am J Emerg Med. 2018;36(11):2058-2060. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

