Interleukin-1β Stimulates Matrix Metalloproteinase 10 Secretion: A Possible Mechanism in Trophoblast-Dependent Spiral Artery Remodeling
- PMID: 40326797
- PMCID: PMC12054339
- DOI: 10.1096/fj.202402329RR
Interleukin-1β Stimulates Matrix Metalloproteinase 10 Secretion: A Possible Mechanism in Trophoblast-Dependent Spiral Artery Remodeling
Abstract
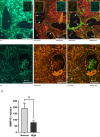
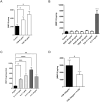
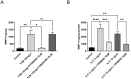
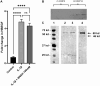
Maternal uterine spiral arteries (SpA) undergo significant structural changes in early pregnancy, resulting in increased blood flow to the developing fetus. Endothelial cells (EC) and vascular smooth muscle cells (VSMC) are lost from the SpA wall and are replaced by trophoblasts. We have previously shown that matrix metalloproteinase 10 (MMP-10) and Heparin binding-EGF like growth factor (HB-EGF) gene expression is increased in a 3D EC/VSMC co-culture system in response to trophoblast secreted factors. This study investigated trophoblast mediated MMP-10 and HB-EGF expression and determined if there was a relationship between the secretion of MMP-10 and the release of soluble HB-EGF (sHB-EGF) from EC. MMP-10 was widely expressed in first trimester decidual tissue including trophoblast, and EC, but not VSMC. MMP-10 expression was significantly lower in decidual tissue from pregnancies at increased risk of developing pre-eclampsia compared to low-risk pregnancies. In vitro, SGHEC-7 cells, a human EC line, but not SGHVMC-9, a human VSMC cell line, secreted MMP-10 in response to trophoblast conditioned medium (TCM). TCM contains several growth factors and cytokines, but only interleukin-1β (IL1β) significantly stimulated MMP-10 secretion by SGHEC-7 cells. Interleukin-1 receptor antagonist (IL-1Ra) significantly inhibited TCM-induced MMP-10 secretion. Interrogation of intracellular pathways established the involvement of MEK and JNK in TCM and IL-1β stimulated MMP-10 secretion. Although IL-1β also significantly increased sHB-EGF, inhibition of MMP-10 activity using a broad spectrum MMP inhibitor had no effect on sHB-EGF. Western blot analysis indicated that MMP-10 secreted by EC in response to IL-1β stimulation was the enzymatically inactive pro form.
Keywords: MMP‐10 (MMP10); endothelial; spiral artery; stromelysin‐2; trophoblast.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have nothing to report.
The authors declare no conflicts of interest.
Figures

References
-
- Smith S. D., Choudhury R. H., Matos P., et al., “Changes in Vascular Extracellular Matrix Composition During Decidual Spiral Arteriole Remodeling in Early Human Pregnancy,” Histology and Histopathology 31 (2016): 557–571. - PubMed
-
- Craven C. M., Morgan T., and Ward K., “Decidual Spiral Artery Remodelling Begins Before Cellular Interaction With Cytotrophoblasts,” Placenta 19 (1998): 241–252. - PubMed
-
- Kam E. P., Gardner L., Loke Y. W., and King A., “The Role of Trophoblast in the Physiological Change in Decidual Spiral Arteries,” Human Reproduction 14 (1999): 2131–2138. - PubMed
-
- Pijnenborg R., Vercruysse L., and Hanssens M., “The Uterine Spiral Arteries in Human Pregnancy: Facts and Controversies,” Placenta 27 (2006): 939–958. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

