Comparative Analysis of Early COVID-19 Treatment Efficacy in a Multicentric Regional Cohort in Italy: Emulation of a Series of Target Trials
- PMID: 40326978
- PMCID: PMC12054396
- DOI: 10.1002/jmv.70379
Comparative Analysis of Early COVID-19 Treatment Efficacy in a Multicentric Regional Cohort in Italy: Emulation of a Series of Target Trials
Abstract
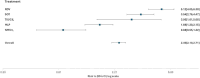
Studies comparing all available strategies for the early treatment of mild-to-moderate COVID-19 during the Omicron era are lacking. We included people with mild-to-moderate COVID-19 and at high risk of progressing to severe disease attending five outpatient clinics in Italy over 2022-2023. The primary outcome was the proportion of participants who experienced Day-30 hospitalization due to COVID-19 or death. Participants received either nirmatrelvir/ritonavir (NMV/r), molnupiravir (MLP), remdesivir (RDV), sotrovimab (SOT), or tixagevimab/cilgavimab (TIX/CIL). We included 10 038 individuals: females 5052 (50%), median age 71 years (IQR 59-81). In total, 1919 (19%) received SOT, 3732 (37.2%) MLP, 1444 (14%) RDV, 2510 (25%) NMV/r, and 433 (4%) TIX/CIL. Only 1689 (17%) had incomplete vaccination, and 2435 (24.3%) were not immunocompetent. The rate of hospitalization/death was 2.40% (95% CI 2.10-2.71). Unadjusted rates were 0.88% (95% CI 0.55-1.32) for NMV/r, 1.69% (95% CI 1.30-2.15) for MLP, 3.0% (95% CI 1.61-5.08) for TIX/CIL, 3.54% (95% CI 2.76-4.47) for SOT and 5.12% (95% CI 4.05-6.39) for RDV. Weighted analysis showed that NMV/r and MLP were superior to all other interventions. In our population of individuals at high risk of progression to severe disease, there was clinical benefit in using NMV/r or MLP instead of mAbs-based therapies or RDV.
Keywords: SARS coronavirus; antiviral agents; epidemiology; virus classification.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
Alessandro Cozzi‐Lepri received research grants or contract from Icona Foundation Study (money paid to UCL) and European Union [Title: “EuCARE: European Cohorts of Patients and Schoolsto Advance Response to Epidemics.” Grant Agreement No. 101046016 (money paid toUCL). Valentina Mazzotta received institutional research grant from Gilead Science, speaking honorariafor congress from ViiV Healthcare e consultation fees for Viatris and Gilead Science. Valentina Siciliano declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events by Pfizer Support for attending meetings by Astrazeneca. Simone Lanini declares consulting fees by GSK, ViiV, MSD and Gilead Support for attending meetings by MSD, Gilead, ViiV. Silvia Meschi declares receipt of equipment, materials, drugs, medical writing, gifts or other services by Abbott srl. Alessandra Vergori declares consulting fees by Gilead Sciences ViiV Healthcare Support for attending meetings by MSD, Gilead, ViiV, Astrazeneca. Enrico Girardi declares Grants or contracts from any entity (if not indicated in item #1 above) by Gilead (paid to INMI) and Mylan (paid to INMI) payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events by Gilead. Loredana Sarmati declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events by Merck, Gilead, Abbvie, Angelini, Astra Zeneca, GSK Support for attending meetings and/or travel by Gilead, Merck, Pfizer. Emanuele Nicastri declares consulting fees from Gliead Science payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events by Roche Support for attending meetings and/or travel by Pharmamar Participation on a Data Safety Monitoring Board or Advisory Board by Takeda and Valneva. Andrea Antinori declares Grants or contracts from any entity (if not indicated in item #1 above) by Gilead (paid to INMI) Astra Zeneca (paid to INMI) ViiV Healthcare (paid to INMI) Consulting fees from Gilead, Astra Zeneca, Pfizer, Janssen‐Cilag, Moderna, GSK, Merck, ViiV Healthcare Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events by Gilead, Moderna, Pfizer, Merck, ViiV Healthcare support for attending meetings and/or travel by Gilead, Astra Zeneca. The other authors declare no conflicts of interest.
Figures

References
-
- Montgomery H., Hobbs F. D. R., Padilla F., et al., “Efficacy and Safety of Intramuscular Administration of Tixagevimab‐Cilgavimab for Early Outpatient Treatment of COVID‐19 (TACKLE): A Phase 3, Randomised, Double‐Blind, Placebo‐Controlled Trial,” Lancet Respiratory Medicine 10, no. 10 (2022): 985–996, 10.1016/S2213-2600(22)00180-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

