Exercise Recommendations and Practical Considerations for Asthma Management-An EAACI Position Paper
- PMID: 40327018
- PMCID: PMC12186601
- DOI: 10.1111/all.16573
Exercise Recommendations and Practical Considerations for Asthma Management-An EAACI Position Paper
Abstract
Exercise is an important treatment for people with asthma and should be considered alongside pharmacological therapy when developing personalised asthma management plans. Despite this, there remains limited guidance concerning the practicalities of asthma-specific exercise prescription. This European Academy of Allergy and Clinical Immunology task force was therefore established to achieve three fundamental aims: first, to provide an up-to-date perspective concerning the role of exercise for asthma management (i.e., describe the disease modifying potential of exercise and associated impact on asthma-related extrapulmonary comorbidities); second, to develop pragmatic recommendations to facilitate safe and effective exercise prescription; and third, to identify key unmet needs and provide focused direction for future research. The position paper is structured as a practically focused document, with recommendations formulated according to best available scientific evidence and expert opinion, with an emphasis on providing healthcare providers with pragmatic advice that can be implemented during routine asthma review.
Keywords: asthma; bronchoconstriction; exercise; management; physical activity; rehabilitation.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
O.J.P. confirms responsibility for the content of the manuscript on behalf of all EAACI task force members.
O.J.P. reports grants from Merck and AstraZeneca outside the submitted work. D.A.A. reports grants from the Spanish Society of Allergology and Clinical Immunology (SEAIC), consulting fees from ALK‐Abelló, AstraZeneca, Chiesi, and Gebro, and speaker fees from AstraZeneca, Chiesi, Gebro, GlaxoSmithKline, Leti Pharma, Menarini, Novartis, Roxall, and Sanofi, all outside the submitted work. S.D.G. reports grants, advisory board, and speaker fees from AstraZeneca, Chiesi, GSK, CSL‐Behring, Novartis, Sanofi, and Takeda, outside the submitted work. V.M.M. reports grants from GlaxoSmithKline and advisory board and speaker fees from GlaxoSmithKline, Menarini, and Boehringer Ingelheim outside the submitted work. M.B. reports grants, advisory board, and speaker fees from AstraZeneca, Chiesi, Grifols, GlaxoSmithKline, Lallemand, Lusofarmaco, Menarini, Omron, and Sanofi outside the submitted work. N.G.P., Vibeke Backer, Valérie Bougault, R.G., I.E.G., E.H., C.J., A.M., and A.S. have declare no conflicts of interest.
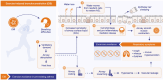
Figures


References
-
- GBD Chronic Respiratory Disease Collaborators , “Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990‐2017: A Systematic Analysis for the Global Burden of Disease Study 2017,” Lancet Respiratory Medicine 8, no. 6 (2020): 585–596, 10.1016/S2213-2600(20)30105-3. - DOI - PMC - PubMed
-
- Global Initiative for Asthma , “2024 GINA Main Report,” 2024, https://ginasthma.org/2024‐report/.
-
- Reddel H. K., FitzGerald J. M., Bateman E. D., et al., “GINA 2019: A Fundamental Change in Asthma Management: Treatment of Asthma With Short‐Acting Bronchodilators Alone Is No Longer Recommended for Adults and Adolescents,” European Respiratory Journal 53, no. 6 (2019): 1901046, 10.1183/13993003.01046-2019. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

