A clinical chemical atlas of xenobiotic toxicity for the Sprague-Dawley rat
- PMID: 40327081
- PMCID: PMC12185569
- DOI: 10.1007/s00204-025-04008-0
A clinical chemical atlas of xenobiotic toxicity for the Sprague-Dawley rat
Abstract
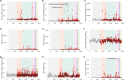
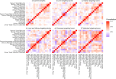
The Consortium for Metabonomic Toxicology (COMET) studies was designed to model metabolic responses to organ- and mechanism-specific toxins to predict acute drug toxicity in rats. A range of clinical chemical parameters were measured in 7-day toxicology studies for 86 toxins eliciting a range of organ- and mechanism-specific effects. Additionally, 21 surgical or physiological stressors were evaluated to identify physiological or metabolic responses that might confound the interpretation of observed toxicity profiles. From these studies on a total of 3473 rats measured at six pharmaceutical companies, we provide a set of 12 serum and 5 urine physical and clinical chemistry parameters. Samples were collected at 24 h, 48 h and 168 h post-dose for each animal and are presented as a downloadable database file. We also summarise the main observations based on the group response at the level of the individual toxin. We demonstrate that correlations between parameters, such as serum bilirubin and aspartate aminotransferase (AST), provide a more nuanced profile of organ-specific toxicity than consideration of individual parameters alone. In addition, we highlight the variability in the measured parameters across the dataset attributable to inter-laboratory differences, and the heterogeneity of metabolic responses to particular compounds or differences in temporal patterns of response. This clinical chemistry atlas of toxicity serves as a valuable reference tool for evaluating the potential toxicity of novel drug candidates.
Keywords: Biofluid; COMET project; Clinical chemistry; Rats; Sprague–Dawley; Toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no conflicts of interest. Ethical approval: All animal studies were conducted in accordance with the current guidelines for animal welfare (Guide for the Care and Use of Laboratory Animals, 1996) and the procedures used were reviewed and approved by the Institutional Animal Care and Use Committee in each company. Consent to participate: Not applicable. Consent for publication: All authors reviewed and approved the final manuscript.
Figures

References
-
- Bollard ME, Contel NR, Ebbels TM, Smith L, Beckonert O, Cantor GH, Lehman-McKeeman L, Holmes EC, Lindon JC, Nicholson JK, Keun HC (2010) NMR-based metabolic profiling identifies biomarkers of liver regeneration following partial hepatectomy in the rat. J Proteome Res 9(1):59–69. 10.1021/pr900200v - DOI - PubMed
-
- Cantor GH, Beckonert O, Bollard ME, Keun HC, Ebbels TM, Antti H, Wijsman JA, Bible RH, Breau AP, Cockerell GL, Holmes E, Lindon JC, Nicholson JK (2013) Integrated histopathological and urinary metabonomic investigation of the pathogenesis of microcystin-LR toxicosis. Vet Pathol 50(1):159–171. 10.1177/0300985812443839 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

