In Situ Polymerization in COF Boosts Li-Ion Conduction in Solid Polymer Electrolytes for Li Metal Batteries
- PMID: 40327199
- PMCID: PMC12055722
- DOI: 10.1007/s40820-025-01768-3
In Situ Polymerization in COF Boosts Li-Ion Conduction in Solid Polymer Electrolytes for Li Metal Batteries
Abstract
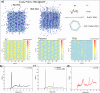
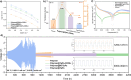
Solid polymer electrolytes (SPEs) have garnered considerable interest in the field of lithium metal batteries (LMBs) owing to their exceptional mechanical strength, excellent designability, and heightened safety characteristics. However, their inherently low ion transport efficiency poses a major challenge for their application in LMBs. To address this issue, covalent organic framework (COF) with their ordered ion transport channels, chemical stability, large specific surface area, and designable multifunctional sites has shown promising potential to enhance lithium-ion conduction. Here, we prepared an anionic COF, TpPa-COOLi, which can catalyze the ring-opening copolymerization of cyclic lactone monomers for the in situ fabrication of SPEs. The design leverages the high specific surface area of COF to facilitate the absorption of polymerization precursor and catalyze the polymerization within the pores, forming additional COF-polymer junctions that enhance ion transport pathways. The partial exfoliation of COF achieved through these junctions improved its dispersion within the polymer matrix, preserving ion transport channels and facilitating ion transport across COF grain boundaries. By controlling variables to alter the crystallinity of TpPa-COOLi and the presence of -COOLi substituents, TpPa-COOLi with partial long-range order and -COOLi substituents exhibited superior electrochemical performance. This research demonstrates the potential in constructing high-performance SPEs for LMBs.
Keywords: Covalent organic framework; In situ polymerization; Lithium metal batteries; Ring-opening polymerization; Solid polymer electrolyte.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Z. Xue, D. He, X. Xie, Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 3(38), 19218–19253 (2015). 10.1039/c5ta03471j - DOI
LinkOut - more resources
Full Text Sources
