One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis
- PMID: 40327280
- PMCID: PMC12085339
- DOI: 10.1007/s12325-025-03183-x
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis
Abstract
Introduction: This study used network meta-analysis (NMA) to evaluate the comparative efficacy of available advanced therapies for moderately to severely active Crohn's disease (CD) versus the IL-23 inhibitor guselkumab.
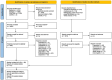
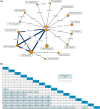
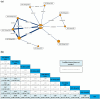
Methods: A systematic literature review was conducted to identify randomized controlled trials (RCTs) of advanced therapies in moderately to severely active CD. Bayesian NMAs were conducted for outcomes of clinical response, clinical remission, endoscopic response, and a combined outcome of clinical remission with endoscopic response, at the end of the maintenance phase (up to 1 year). Primary analyses included patients with varied prior inadequate treatment responses, with additional analyses conducted for specific subgroups. Re-randomized trials were normalized in several cases to mimic a standard treat-through design, incorporating data from additional sources, when necessary, for patients who had an inadequate response or experienced a delayed response following induction.
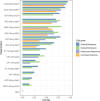
Results: Of the 58 RCTs identified, 13 with maintenance endpoint data were ultimately included in the NMAs. Guselkumab 100 mg and 200 mg were more likely to be effective versus several comparators. Guselkumab 200 mg demonstrated significantly greater efficacy versus infliximab 10 mg/kg every 8 weeks and upadacitinib 30 mg daily for clinical response and clinical remission. For endoscopic response, guselkumab 200 mg showed significantly greater efficacy than ustekinumab, adalimumab, and upadacitinib. Significance was also noted versus ustekinumab on the combined outcome of clinical remission with endoscopic response. Similarly, guselkumab 100 mg demonstrated efficacy versus comparators across analyses. Guselkumab achieved higher rankings based on surface under the cumulative ranking curve. Findings of primary analyses within mixed populations were generally corroborated by subpopulation analyses.
Conclusion: Results of this NMA in moderately to severely active CD indicate a higher likelihood of guselkumab achieving each clinical and endoscopic endpoint analyzed at the end of the maintenance phase versus other advanced therapies assessed.
Keywords: Biologics; Crohn’s disease; Guselkumab; Indirect treatment comparisons; Network meta-analysis; Study design.
Plain language summary
A network meta-analysis (NMA) was completed to compare the effectiveness of advanced treatments for Crohn’s disease, a chronic inflammatory condition of the digestive tract. Our NMA focused on the drug guselkumab and how effective it is compared with other treatments for Crohn’s disease. We looked at 4 outcomes related to efficacy that are common in trials: clinical response (improvement in symptoms), clinical remission (disappearance of symptoms), endoscopic response (seen in the digestive tract), and a combined outcome of clinical remission with endoscopic response. Data were analyzed from trials lasting up to 1 year. Patients who had not responded well to prior treatments were included in the analysis. Thirteen trials met all criteria and were included in the analysis. Guselkumab at doses of 100 mg and 200 mg was more effective than several treatments. Guselkumab 200 mg was significantly better than infliximab and upadacitinib for both clinical response and clinical remission. It also showed better results than ustekinumab, adalimumab, and upadacitinib for endoscopic response and was more effective than ustekinumab for the combined outcome of clinical remission with endoscopic response. Our analysis shows that guselkumab is likely to be more effective at achieving both symptom improvement and healing the digestive tract compared with other advanced treatments for moderate to severe Crohn’s disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Tim Disher, Meaghan Bartlett, Becky Hooper, Jenna Ellis, and Ashley Bonner are current or former employees of EVERSANA. Myrlene Sanon, Dominik Naessen, and Zijiang Yang are employees of Johnson & Johnson and may hold stock/stock options in Johnson & Johnson. Drs. Axel Dignass and Jessica R. Allegretti have received personal compensation from Janssen for consultant fees. Axel Dignass reports fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis, and endpoint committees from Abivax, AbbVie, Bristol Myers Squibb, Dr Falk Pharma, Galapagos, Gilead, Janssen, and Pfizer; consultancy fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Dr Falk Foundation, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, J&J, Lilly, MSD, Pfizer, Pharmacosmos, Sandoz, Stada, Takeda, Tillotts, and Vifor Pharma; payment from lectures including service on speakers bureaus from AbbVie, Biogen, CED Service GmbH, Celltrion, Falk Foundation, Ferring, Galapagos, Gilead, High5MD, Janssen, Materia Prima, MedToday, MSD, Pfizer, Streamed-Up, Takeda, Tillotts, and Vifor Pharma; and payment for manuscript preparation from Falk Foundation, Takeda, Thieme, and UniMed Verlag. Preparation of this manuscript was funded by Janssen. Ethical Approval: This analysis and summary are based on previously completed/published trials and do not contain any new studies with human participants or animals performed by any of the authors.
Figures


References
-
- Veauthier B, Hornecker JR. Crohn’s disease: diagnosis and management. Am Fam Physician. 2018;98:661–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

