Chemical and Electrochemical Investigation of the Oxidation of a Highly Reduced Fe6C Iron Carbide Carbonyl Cluster: A Synthetic Route to Heteroleptic Fe6C and Fe5C Clusters
- PMID: 40327361
- PMCID: PMC12093381
- DOI: 10.1021/acs.inorgchem.5c01014
Chemical and Electrochemical Investigation of the Oxidation of a Highly Reduced Fe6C Iron Carbide Carbonyl Cluster: A Synthetic Route to Heteroleptic Fe6C and Fe5C Clusters
Abstract
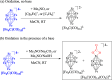
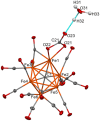
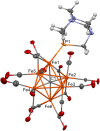
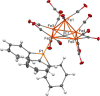
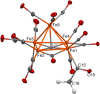
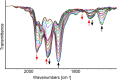
A chemical and electrochemical investigation of the redox chemistry of [Fe6C(CO)15]4- is reported and supported by computational studies. Depending on the experimental conditions, the original Fe6C cage is retained or partially degraded to Fe5C. Chemical oxidation of [Fe6C(CO)15]4- with [Cp2Fe][PF6], [C7H7][BF4], or Me3NO affords the previously reported [Fe6C(CO)16]2-, whereas oxidation in the presence of a base (Na2CO3 or NaOH) results in the new carbonate-carbide cluster [Fe6C(CO)14(CO3)]4-. Oxidation of [Fe6C(CO)15]4- in the presence of a phosphine ligand produces the heteroleptic species [Fe6C(CO)15(PTA)]2- and [Fe5C(CO)13(PPh3)]2-. Reaction of [Fe6C(CO)15]4- with alkylating or acylating agents (MeI, CF3SO3Me, and MeCOCl) affords the acetyl-carbide cluster [Fe5C(CO)13(COMe)]3-, with partial oxidative degradation of the original Fe6C cage. The new clusters have been spectroscopically and structurally characterized. The redox chemistry of [Fe6C(CO)15]4- was further investigated by electrochemical and spectroelectrochemical methods. According to computational outcomes, the spectroelectrochemical oxidation of [Fe6C(CO)15]4- follows an EEC mechanism, leading to the formation of [Fe6C(CO)16]2-. The [Fe6C(CO)15]3- intermediate can accumulate and be spectroscopically detected. These new chemical and electrochemical findings have been supported and corroborated by computational methods. DFT calculations suggest an EEC pathway also for the reverse electrochemical process, i.e., reduction of [Fe6C(CO)16]2- to [Fe6C(CO)15]4-.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




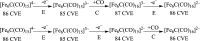
References
-
- Braye E. H.; Dahl L. F.; Hübel W.; Wampler D. L. The Preparation, Properties and Structure of the Iron Carbonyl Carbide Fe5(CO)15C. J. Am. Chem. Soc. 1962, 84, 4633–4639. 10.1021/ja00883a004. - DOI
-
- Cesari C.; Femoni C.; Iapalucci M. C.; Zacchini S. Molecular Fe, Co, and Ni carbide carbonyl clusters and nanoclusters. Inorg. Chim. Acta 2023, 544, 12123510.1016/j.ica.2022.121235. - DOI
-
- Churchill M. R.; Wormald J.; Knight J.; Mays M. J. Synthesis and Crystallographic Characterization of [Me4N+]2[Fe6(CO)16C2–], a Hexanuclear Carbidocarbonyl Derivative of Iron. J. Am. Chem. Soc. 1971, 93, 3073–3074. 10.1021/ja00741a058. - DOI
-
- Bortoluzzi M.; Ciabatti I.; Cesari C.; Femoni C.; Iapalucci M. C.; Zacchini S. Synthesis of the Highly Reduced [Fe6C(CO)15]4– Carbonyl Carbide Cluster and Its Reactions with H+ and [Au(PPh3)]+. Eur. J. Inorg. Chem. 2017, 2017, 3135–3143. 10.1002/ejic.201700169. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

