A spontaneous nonhuman primate model of inherited retinal degeneration
- PMID: 40327408
- PMCID: PMC12220943
- DOI: 10.1172/jci.insight.190807
A spontaneous nonhuman primate model of inherited retinal degeneration
Abstract
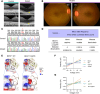
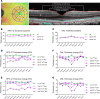
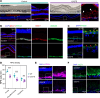
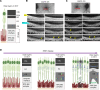
Inherited retinal degenerations (IRDs) are important causes of progressive, irreversible blindness. Hereditary macular diseases, in particular, are significant in their effect on the specialized, central cone photoreceptor-rich macula responsible for high resolution vision. Autosomal dominant Best vitelliform macular dystrophy (BVMD), caused by variants in the BEST1 gene, is one of the most common inherited macular dystrophies. Gene therapies have emerged as promising treatments for IRDs, but a lack of suitable animal models has hindered progress both in treatments and in understanding the mechanisms underlying macular diseases. Here, we report a Macaca fascicularis carrying a heterozygous potential pathogenic BEST1p.Q327E variant that disrupts the BEST1 ion channel by destabilizing the A195 helix, mirroring the structural perturbations seen in certain human pathological mutants. Longitudinal imaging over 2 years revealed progressive macular changes, including subfoveal cleft enlargement, lipid-rich deposit accumulation, retinal pigment epithelium (RPE) disruption, and central-to-peripheral photoreceptor degeneration, recapitulating early human BVMD pathology. Histopathology demonstrated diminished BEST1 expression, attenuation of the RPE-photoreceptor interface, and 2 distinct types of lipid deposits, including heretofore unappreciated cone mitochondrial-enriched lesions, highlighting selective cone mitochondria vulnerability. This is, to our knowledge, the first nonhuman primate model of inherited macular dystrophy, and it links BEST1 mutations, mitochondrial dysfunction, and progressive macular degeneration, offering new insights into BVMD pathophysiology and highlighting its utility for studying disease progression and potential therapeutic interventions.
Keywords: Genetic diseases; Genetics; Mitochondria; Ophthalmology; Retinopathy.
Figures


References
-
- Tsang SH, Sharma T. Best vitelliform macular dystrophy. Adv Exp Med Biol. 2018;1085:157–158. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

