Shc1 cooperates with Frs2 and Shp2 to recruit Grb2 in FGF-induced lens development
- PMID: 40327534
- PMCID: PMC12055001
- DOI: 10.7554/eLife.103615
Shc1 cooperates with Frs2 and Shp2 to recruit Grb2 in FGF-induced lens development
Abstract
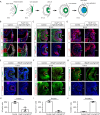
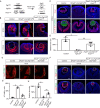
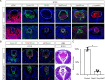
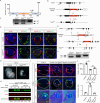
Fibroblast growth factor (FGF) signaling elicits multiple downstream pathways, most notably the Ras/MAPK cascade facilitated by the adaptor protein Grb2. However, the mechanism by which Grb2 is recruited to the FGF signaling complex remains unresolved. Here, we showed that genetic ablation of FGF signaling prevented murine lens induction by disrupting transcriptional regulation and actin cytoskeletal arrangements, which could be reproduced by deleting the juxtamembrane region of the FGF receptor and rescued by Kras activation. Conversely, mutations affecting the Frs2-binding site on the FGF receptor or the deletion of Frs2 and Shp2 primarily impact later stages of lens vesicle development involving lens fiber cell differentiation. Our study further revealed that the loss of Grb2 abolished MAPK signaling, resulting in a profound arrest of lens development. However, removing Grb2's putative Shp2 dephosphorylation site (Y209) neither produced a detectable phenotype nor impaired MAPK signaling during lens development. Furthermore, the catalytically inactive Shp2 mutation (C459S) only modestly impaired FGF signaling, whereas replacing Shp2's C-terminal phosphorylation sites (Y542/Y580) previously implicated in Grb2 binding only caused placental defects, perinatal lethality, and reduced lacrimal gland branching without impacting lens development, suggesting that Shp2 only partially mediates Grb2 recruitment. In contrast, we observed that FGF signaling is required for the phosphorylation of the Grb2-binding sites on Shc1 and the deletion of Shc1 exacerbates the lens vesicle defect caused by Frs2 and Shp2 deletion. These findings establish Shc1 as a critical collaborator with Frs2 and Shp2 in targeting Grb2 during FGF signaling.
Keywords: FGF; Grb2; MAPK; Shc; Shp2; cell biology; developmental biology; lens induction; mouse.
Plain language summary
Cells communicate by releasing proteins that bind to receptors on recipient cells, triggering a cascade of events that alter the cell’s behavior. A family of signaling proteins called fibroblast growth factors (FGFs) is critical for various biological processes, especially during embryonic development. While scientists have a good understanding of how FGFs reach their target cells, less is known about the series of events they activate once they bind to a receptor. Three adaptor proteins – called Frs2, Shp2 and Grb2 – are essential for propagating the FGF signal. First, the activated receptor binds to and adds phosphate groups to Frs2, which then recruits and facilitates the phosphorylation of Shp2 and Grb2. Here, Wang, Li, Mao et al. offer fresh insights into how this complex of molecules transmit the FGF signal through cells during lens development. First, the team genetically modified the structure and activity of FGF receptors in mice to see how this impacted the formation of their lenses. They found that the membrane-embedded portion of the receptor, which includes the binding site for Frs2, is critical for regulating the consecutive steps of lens development. However, the initial stages of lens formation could still occur when only the Frs2 binding site was mutated. Loss of Grb2 produced a similar effect, suggesting that Frs2 and Grb2 are particularly important for the later stages of lens development. Previous studies have suggested that Shp2 acts as a bridge between Frs2 and Grb2. To test this theory, Wang, Li, Mao et al. deleted the two sites in Shp2 that are responsible for binding to Grb2 and stopped phosphorylation interactions between the two adaptors. While these changes affected embryo survival, they had only a modest impact on lens development. Further experiments revealed that another adaptor protein called Shc1 can also mediate Grb2 recruitment and activation, and may be responsible for transmitting the FGF signal later in lens development. This study provides deeper insights into the network of signaling molecules activated by FGFs, uncovering new mechanisms and adaptors involved in this pathway. The findings suggest that the FGF signaling network is highly adaptable, with different components being required at specific stages of development. Future research expanding on this work may lead to the discovery of therapies that target specific organs affected by FGF-related disorders.
© 2024, Wang, Li, Mao et al.
Conflict of interest statement
QW, HL, YM, AG, EP, YW, AC, JP, XZ No competing interests declared
Figures





Update of
-
Shc1 cooperates with Frs2 and Shp2 to recruit Grb2 in FGF-induced lens development.bioRxiv [Preprint]. 2025 Feb 17:2024.10.20.619055. doi: 10.1101/2024.10.20.619055. bioRxiv. 2025. Update in: Elife. 2025 May 06;13:RP103615. doi: 10.7554/eLife.103615. PMID: 39484547 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

