Effectiveness over time of a primary series of the original monovalent COVID-19 vaccines in adults in the United States
- PMID: 40327641
- PMCID: PMC12054878
- DOI: 10.1371/journal.pone.0320434
Effectiveness over time of a primary series of the original monovalent COVID-19 vaccines in adults in the United States
Abstract
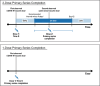
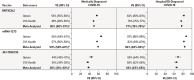
With data from 2 US claims databases (Optum, CVS Health) supplemented with Immunization Information System COVID-19 vaccine records, we evaluated overall and time-specific vaccine effectiveness (VE) of an initial primary series for 3 monovalent COVID-19 vaccines-BNT162b2, mRNA-1273, and JNJ-7836735-in adults (18-64 years). Vaccinated individuals were matched to unvaccinated comparators, and we estimated VE against any medically diagnosed COVID-19 and hospital/emergency department (ED)-diagnosed COVID-19. Additionally, we estimated VE by era of predominant variants, in subgroups, and compared across vaccine brands. The cohorts consisted of 341,097 (Optum) and 1,151,775 (CVS Health) matched pairs for BNT162b2; 201,604 (Optum) and 651,545 (CVS Health) for mRNA-1273; and 49,285 (Optum) and 149,813 (CVS Health) for JNJ-7836735. The study period began 11 December 2020 (date of first COVID-19 vaccine availability in the US) and ended 15 January 2022 in Optum and 31 March 2022 in CVS Health. Summary VE estimates from meta-analysis against hospital/ED-diagnosed COVID-19 were: BNT162b2, 77% (95% CI, 76%-78%); mRNA-1273, 84% (95% CI, 83%-85%), JNJ-7836735 66% (95% CI, 63%-68%). VE estimates were higher for hospital/ED-diagnosed COVID-19 than for medically diagnosed COVID-19, and VE estimates were highest in adults receiving mRNA-1273 for both outcomes. VE was sustained for approximately 7 months for medically diagnosed and up to 9 months for hospital/ED-diagnosed COVID-19. VE differed by brand and variant era. Ongoing real-world surveillance of COVID-19 vaccines using robust data sources and methodology is needed as new variants and recommendations for updated vaccines have evolved.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: JBL, CB, DI, MMR, and MSA are employees of RTI International, an independent, nonprofit research institute that performs research on behalf of governmental and commercial clients, including vaccine manufacturers. LP, JD, RO, RP, MM, JS, LBW, GWY, JS, and KA are employees of Optum and own stock in UnitedHealth Group. EJB is an employee of Optum. DAD and CNMW are employees of CVS Health. The other authors have no conflicts to report.
Figures


References
-
- Bajema KL, Dahl RM, Prill MM, Meites E, Rodriguez-Barradas MC, Marconi VC, et al. Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalization - Five Veterans Affairs Medical Centers, United States, February 1-August 6, 2021. MMWR Morb Mortal Wkly Rep. 2021 Sep 17;70(37):1294-9. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

