Naturally ornate RNA-only complexes revealed by cryo-EM
- PMID: 40328315
- PMCID: PMC12286853
- DOI: 10.1038/s41586-025-09073-0
Naturally ornate RNA-only complexes revealed by cryo-EM
Abstract
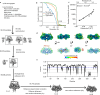
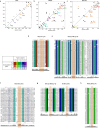
The structures of natural RNAs remain poorly characterized and may hold numerous surprises1-4. Here we report three-dimensional structures of three large ornate bacterial RNAs using cryo-electron microscopy (cryo-EM). GOLLD (Giant, Ornate, Lake- and Lactobacillales-Derived), ROOL (Rumen-Originating, Ornate, Large) and OLE (Ornate Large Extremophilic) RNAs form homo-oligomeric complexes whose stoichiometries are retained at lower concentrations than measured in cells. OLE RNA forms a dimeric complex with long co-axial pipes spanning two monomers. Both GOLLD and ROOL form distinct RNA-only multimeric nanocages with diameters larger than the ribosome, each empty except for a disordered loop. Extensive intramolecular and intermolecular A-minor interactions, kissing loops, an unusual A-A helix and other interactions stabilize the three complexes. Sequence covariation analysis of these large RNAs reveals evolutionary conservation of intermolecular interactions, supporting the biological importance of large, ornate RNA quaternary structures that can assemble without any involvement of proteins.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

