Rationale and design of a randomised phase II multicentre crossover trial investigating a sodium-glucose co-transporter 2 inhibitor, dapagliflozin, combined with a novel continuous ketone monitor in adults with type 1 diabetes to reduce the risk of diabetic ketoacidosis: the PARTNER study
- PMID: 40328646
- PMCID: PMC12056658
- DOI: 10.1136/bmjopen-2024-098457
Rationale and design of a randomised phase II multicentre crossover trial investigating a sodium-glucose co-transporter 2 inhibitor, dapagliflozin, combined with a novel continuous ketone monitor in adults with type 1 diabetes to reduce the risk of diabetic ketoacidosis: the PARTNER study
Abstract
Introduction: Sodium-glucose co-transporter inhibitors have potential glycaemic and non-glycaemic benefits in people with type 1 diabetes (T1D). However, the increased risk of diabetic ketoacidosis (DKA) limits their widespread use. We hypothesise that dapagliflozin 10 mg daily, combined with the use of continuous ketone monitoring (CKM) and education strategies to mitigate progression to DKA, will demonstrate improved glycaemic control without increasing DKA events.
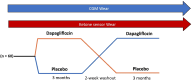
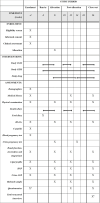
Methods and analysis: PARTNER is a multisite 6-month randomised crossover double-masked study involving Australian adults with T1D who have a Haemoglobin A1c (HbA1c) <85.8 mmol/mol (<10%), minimum total daily insulin dose ≥0.4 IU/kg, consume ≥100 g carbohydrates/day and have not had DKA in the last 3 months. All participants will undergo a 2-week run-in period wearing the Abbott FreeStyle Libre 2 Continuous Glucose Monitor (CGM) and Abbott CKM device. Following this, participants are randomised to receive dapagliflozin or placebo for 12 weeks, followed by crossover for a further 12 weeks separated by a 2-week washout period. The primary effectiveness outcome is the Abbott FreeStyle Libre 2 CGM time in range during the final 2 weeks of each stage. The primary safety outcome is the number of episodes of DKA requiring hospitalisation or emergency department presentation. 60 participants will be recruited across five sites.
Ethics and dissemination: The study has received ethical approval from the St Vincent's Hospital Melbourne Human Research Ethics Committee (HREC reference 302/23). The results will be published in peer-reviewed journals and presented at national and international diabetes conferences.
Trial registration number: ACTRN12624000448549.
Keywords: Clinical Protocols; Clinical trials; DIABETES & ENDOCRINOLOGY.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: DNO'N has received honoraria from Medtronic, Insulet, Abbott, Novo and Sanofi; and Research Support from Medtronic, Insulet, Dexcom, Roche, GlySense, BioCapillary and Endogenex; and are on Advisory Boards for Medtronic, Insulet, Abbott, Ypsomed, Novo, and Sanofi EE’s institution has received research funding from Eli Lilly, Boehringer-Ingelheim, Novo Nordisk, Pharmasol. EE has received funding for honoraria for speakers and advisory fees from Abbott, Bayer, Sanofi and the money received is directly donated to EE’s institution for diabetes research. SF has received honoraria for speaker fees for AstraZeneca, Boehringer-Ingelheim, Lilly, Novo Nordisk and honoraria for advisor fees for Medtronic, Mylan, Pfizer, Sanofi. ST reports nonfinancial support from Abbott Diabetes. AJ’s institutions have received research funding from Medtronic, Insulet, Ypsomed, GlySense and Endogenex and is on Advisory Boards of Abbott (Diabetes, Australia) and GSK. All other authors have no competing interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
