Cohort profile: the Swedish Inception Cohort in inflammatory bowel disease (SIC-IBD)
- PMID: 40328654
- PMCID: PMC12056626
- DOI: 10.1136/bmjopen-2025-099218
Cohort profile: the Swedish Inception Cohort in inflammatory bowel disease (SIC-IBD)
Abstract
Purpose: There is a need for diagnostic and prognostic biosignatures to improve long-term outcomes in inflammatory bowel disease (IBD). Here, we describe the establishment of the Swedish Inception Cohort in IBD (SIC-IBD) and demonstrate its potential for the identification of such signatures.
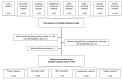
Participants: Patients aged ≥18 years with gastrointestinal symptoms who were referred to the gastroenterology unit due to suspected IBD at eight Swedish hospitals between November 2011 and March 2021 were eligible for inclusion.
Findings to date: In total, 367 patients with IBD (Crohn's disease, n=142; ulcerative colitis, n=201; IBD-unclassified, n=24) and 168 symptomatic controls were included. In addition, 59 healthy controls without gastrointestinal symptoms were recruited as a second control group. Biospecimens and clinical data were collected at inclusion and in patients with IBD also during follow-up to 10 years. Levels of faecal calprotectin and high-sensitivity C-reactive protein were higher in patients with IBD compared with symptomatic controls and healthy controls. Preliminary results highlight the potential of serum protein signatures and autoantibodies, as well as results from faecal markers, to differentiate between IBD and symptomatic controls in the cohort. During the first year of follow-up, 37% (53/142) of the patients with Crohn's disease, 24% (48/201) with ulcerative colitis and 4% (1/24) with IBD-U experienced an aggressive disease course.
Future plans: We have established an inception cohort enabling ongoing initiatives to collect and generate clinical data and multi-omics datasets. The cohort will allow analyses for translation into candidate biosignatures to support clinical decision-making in IBD. Additionally, the data will provide insights into mechanisms of disease pathogenesis.
Keywords: GASTROENTEROLOGY; Inflammatory bowel disease; Prognosis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: DB served as a speaker and/or advisory board member for Abbvie, BMS, Johnson and Johnson, Pfizer, Pharmacosmos, Sandoz, Takeda and Tillotts Pharma. HS has served as speaker and/or advisory board member for AbbVie Ferring, Janssen, Pfizer, Takeda, Gilead and Tillotts Pharma. AC served as a speaker for Takeda and Ferring. HH has served as a speaker, consultant or advisory board member for AbbVie, Ferring, Fresenius Kabi, Janssen, Norgine, Pfizer, Pharmacosmos, Takeda and Tillotts and has received grant support from AbbVie, Ferring, Takeda, and Tillotts. SA served as a member for Scientific committee/Advisory board of, Janssen, Pharmacosmos, Takeda, as a consultant for Janssen, OrionPharm, Takeda, and as a speaker: Galapagos, Janssen, Tillotts, received research grants from Janssen and OrionPharma. FB has acted as national and/or local principal investigator for clinical trials for Janssen, Ferring and Abbvie. CE received grant support/lecture fee/advisory board from Takeda, Janssen Cilag, Pfizer, Abbvie. OGri served as speaker and/or advisory board member for Abbvie, Bristol Mayer Squibb, Eli Lilly, Ferring, Janssen, Pfizer Takeda, Tillotts Pharma and Vifor Pharma. JM has served as a speaker, consultant or advisory board member for AbbVie, Alfasigma, Amgen, Bayer, Biogen, BMS, Eli Lilly, Ferring, Galapagos, Hospira, ITH, Janssen, Lument, MSD, Otsuka, Pfizer, Sandoz, Takeda, Tillotts and UCB; and has received grant support (not for this study) from AbbVie, BMS, Calpro AS, Carbiotix, Ferring, Fresenius Kabi, Pfizer, Svar Life Science and Takeda. MKM has received speaker's fees from Takeda and Janssen. LÖ has received a financial support for research by Genetic Analysis AS, Biocodex, Danone Research and AstraZeneca and served as Consultant/Advisory Board member for Genetic Analysis AS, and as a speaker for Biocodex, Ferring Pharmaceuticals, Takeda, AbbVie, Novartis, Janssen-Cilag and Meda. MC has received speaker's fees from ViforPharma. She is the national PI for clinical trials for AstraZeneca. CRHH has served as a speaker and/or advisory board member for AstraZeneca, Dr Falk Pharma and the Falk Foundation, Galapagos, Janssen, Pfizer, Takeda, Tillotts Pharma, and received grant support from Tillotts and Takeda. JH served as speaker and/or advisory board member from AbbVie, Alfasigma, Aqilion, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma and the Falk Foundation, Eli Lilly, Ferring, Galapagos, Gilead, Hospira, Index Pharma, Janssen, MEDA, Medivir, Medtronic, Merck, MSD, Novartis, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, STADA, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, UCB and received grant support from Janssen, MSD and Takeda. The remaining authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
