A highly conserved neuronal microexon in DAAM1 controls actin dynamics, RHOA/ROCK signaling, and memory formation
- PMID: 40328765
- PMCID: PMC12056172
- DOI: 10.1038/s41467-025-59430-w
A highly conserved neuronal microexon in DAAM1 controls actin dynamics, RHOA/ROCK signaling, and memory formation
Abstract
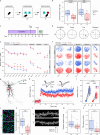
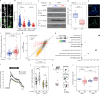
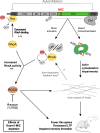
Actin cytoskeleton dynamics is essential for proper nervous system development and function. A conserved set of neuronal-specific microexons influences multiple aspects of neurobiology; however, their roles in regulating the actin cytoskeleton are unknown. Here, we study a microexon in DAAM1, a formin-homology-2 (FH2) domain protein involved in actin reorganization. Microexon inclusion extends the linker region of the DAAM1 FH2 domain, altering actin polymerization. Genomic deletion of the microexon leads to neuritogenesis defects and increased calcium influx in differentiated neurons. Mice with this deletion exhibit postsynaptic defects, fewer immature dendritic spines, impaired long-term potentiation, and deficits in memory formation. These phenotypes are associated with increased RHOA/ROCK signaling, which regulates actin-cytoskeleton dynamics, and are partially rescued by treatment with a ROCK inhibitor. This study highlights the role of a conserved neuronal microexon in regulating actin dynamics and cognitive functioning.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

