CHD8 interacts with BCL11A to induce oncogenic transcription in triple negative breast cancer
- PMID: 40328966
- PMCID: PMC12170886
- DOI: 10.1038/s44318-025-00447-8
CHD8 interacts with BCL11A to induce oncogenic transcription in triple negative breast cancer
Abstract
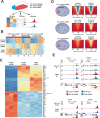
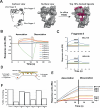
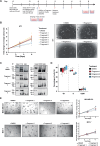
The identification of tumour-specific protein-protein interactions remains a challenge for the development of targeted cancer therapies. In this study we describe our approach for the identification of triple negative breast cancer (TNBC)-specific protein-protein interactions focusing on the oncogene BCL11A. We used a proteomic approach to identify the BCL11A protein networks in TNBC and compared it to its network in B-cells, a cell type in which BCL11A plays crucial roles. This approach identified the chromatin remodeller CHD8 as a TNBC-specific interaction partner of BCL11A. We show that CHD8 also plays a key role in TNBC pathogenesis, with detailed multi-omics analysis revealing that BCL11A and CHD8 co-regulate several targets and synergise to drive tumour development and progression. Using a battery of biophysical assays, we confirm that the BCL11A-CHD8 interaction is direct and identify chemical fragments that disrupt this interaction and affect downstream targets, decreasing proliferation in 3D colony assays. Our study provides a proof-of-principle approach for investigating tumour-specific protein-protein interactions and identifies lead chemical compounds that could be developed into novel therapeutics for TNBC.
Keywords: BCL11A; CHD8; TNBC; Tumour Specific Protein–protein Interactions.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures


References
-
- Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193 - PubMed
-
- Capdeville R, Buchdunger E, Zimmermann J, Matter A (2002) Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov 1:493–502 - PubMed
-
- Carey L, Winer E, Viale G, Cameron D, Gianni L (2010) Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 7:683–692 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

