Gut commensal Phascolarctobacterium faecium retunes innate immunity to mitigate obesity and metabolic disease in mice
- PMID: 40328980
- PMCID: PMC12137122
- DOI: 10.1038/s41564-025-01989-7
Gut commensal Phascolarctobacterium faecium retunes innate immunity to mitigate obesity and metabolic disease in mice
Abstract
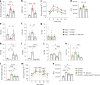
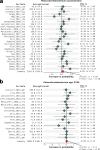
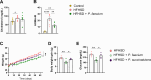
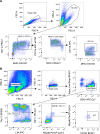
The gut microbiota may protect against obesity and chronic metabolic conditions by regulating the immune response to dietary triggers. Yet the specific bacteria that control the overactivation of the immune system in obesity and their mode of action remain largely unknown. Here we surveyed 7,569 human metagenomes and observed an association between the gut symbiont Phascolarctobacterium faecium and non-obese adults regardless of nationality, sex or age. In a mouse model of diet-induced obesity, we confirmed the specificity of P. faecium DSM 32890 anti-obesogenic properties compared with other species of the same genus. P. faecium reversed the inflammatory phenotype associated with obesity. Specifically, P. faecium promoted polarization of alternatively activated macrophages (M2), which reversed the obesity-induced increase in gut-resident type 1 innate lymphoid cells. This resulted in mitigation of glucose intolerance, adiposity and body weight gain irrespective of treatment with live or pasteurized bacteria. The metabolic benefits were independent of the adaptive immune system, but they were abolished by an inhibitor of M2 polarization in mice. P. faecium directly promoted M2-macrophage polarization through TLR2 signalling and these effects seemed to be independent of gut microbiota changes. Overall, we identify a previously undescribed gut commensal bacterium that could help mitigate obesity and metabolic comorbidities by retuning the innate immune response to hypercaloric diets.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.S., M.R.-P. and I.L.-A. are authors of a patent (patent application no. 201831166, Spain, 2018) belonging to CSIC on Phascolarctobacterium faecium DSM 32890. The other authors declare no competing interests.
Figures







References
-
- World Health Organization. Obesity and overweight. Fact sheetshttps://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 24 January 2023).
-
- Amabebe, E. et al. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr.123, 1127–1137 (2020). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

