Rapid development of a registry to accelerate COVID-19 vaccine clinical trials
- PMID: 40328984
- PMCID: PMC12056171
- DOI: 10.1038/s41746-025-01666-3
Rapid development of a registry to accelerate COVID-19 vaccine clinical trials
Abstract
Response to the SARS-Cov-2 pandemic required the unprecedented, rapid activation of the COVID-19 Prevention Network (CoVPN) representing hundreds of sites conducting vaccine clinical trials. The CoVPN Volunteer Screening Registry (VSR) collected participant information, distributed qualified candidates across sites, and monitored enrollment progress. The system consisted of three web-based interfaces. The Volunteer Questionnaire flowed into a secure database. The Site Portal supported volunteer selection, analytics, and enrollment. The Administrative Portal enabled dynamic analytic reports by geography, clinical trial, and site, including volunteering rates over time. The VSR collected over 650,000 volunteers, serving a key role in the recruitment of diverse participants for multiple Phase 3 clinical trials. Over 47% of the 166,729 volunteers selected for screening represented prioritized groups. The success of the VSR demonstrates how digital tools can be rapidly yet safely integrated into an accelerated clinical trial setting. We summarize the development of the system and lessons learned for pandemic preparedness.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.G.K., N.F.A., K.M., G.B.B., M.T., and L.R. declare no conflicts; R.L., S.S., A.K., D.M., S.M., and J.H. are employed by Oracle Corporation and hold stock options.
Figures
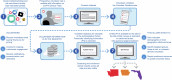

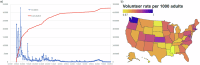
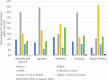
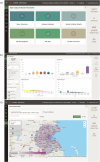
Update of
-
Rapid Development of a Registry to Accelerate COVID-19 Vaccine Clinical Trials.Res Sq [Preprint]. 2024 Jun 10:rs.3.rs-4397271. doi: 10.21203/rs.3.rs-4397271/v1. Res Sq. 2024. Update in: NPJ Digit Med. 2025 May 6;8(1):251. doi: 10.1038/s41746-025-01666-3. PMID: 38947011 Free PMC article. Updated. Preprint.
References
-
- Leroux, H., McBride, S. & Gibson, S. In Health Informatics: The Transformative Power of Innovation 89−95 (IOS Press, 2011).
Grants and funding
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1AI068635/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- UM1AI068614/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Miscellaneous

