Human-machine co-adaptation to automated insulin delivery: a randomised clinical trial using digital twin technology
- PMID: 40329052
- PMCID: PMC12055975
- DOI: 10.1038/s41746-025-01679-y
Human-machine co-adaptation to automated insulin delivery: a randomised clinical trial using digital twin technology
Abstract
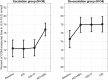
Most automated insulin delivery (AID) algorithms do not adapt to the changing physiology of their users, and none provide interactive means for user adaptation to the actions of AID. This randomised clinical trial tested human-machine co-adaptation to AID using new 'digital twin' replay simulation technology. Seventy-two individuals with T1D completed the 6-month study. The two study arms differed by the order of administration of information feedback (widely used metrics and graphs) and in silico co-adaptation routine, which: (i) transmitted AID data to a cloud application; (ii) mapped each person to their digital twin; (iii) optimized AID control parameters bi-weekly, and (iv) enabled users to experiment with what-if scenarios replayed via their own digital twins. In silico co-adaptation improved the primary outcome, time-in-range (3.9-10 mmol/L), from 72 to 77 percent (p < 0.01) and reduced glycated haemoglobin from 6.8 to 6.6 percent. Information feedback did not have additional effect to AID alone. (Clinical Trials Registration: NCT05610111 (November 10, 2022)).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.P.K. reports research grants handled by the University of Virginia from the National Institutes of Health, Novo Nordisk, Dexcom, and Tandem Diabetes Care. Additionally, B.P.K. has patents with royalties paid to Dexcom and Novo Nordisk, handled by the University of Virginia Licensing and Ventures Group. P.C. is at present an employee of Dexcom, Inc.; the work presented in this article was performed as part of his UVA appointment and is independent of his employment with Dexcom, Inc. J.P. has received research support through his previous institution from Dexcom. J.D.C. is at present an employee of Insulet Corp.; the work presented in this article was performed as part of her UVA appointment and is independent of her employment with Insulet. M.V.T., C.L.K., G.S., C.A. declare no conflicts. M.S. has received support to her institution from Dexcom, Insulet and Tandem Diabetes Care. S.A.B. has received research support through her institution from Dexcom, Insulet, Roche Diagnostics, Tandem Diabetes Care and Tolerion and has participated on a data monitoring board for MannKind.
Figures
References
Associated data
Grants and funding
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
- RO1 DK085623/U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases
LinkOut - more resources
Full Text Sources
Medical