Biologics for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-analysis
- PMID: 40329054
- PMCID: PMC12126413
- DOI: 10.1007/s13555-025-01423-0
Biologics for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-analysis
Erratum in
-
Correction: Biologics for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-analysis.Dermatol Ther (Heidelb). 2025 Oct;15(10):3069-3070. doi: 10.1007/s13555-025-01527-7. Dermatol Ther (Heidelb). 2025. PMID: 40855032 Free PMC article. No abstract available.
Abstract
Introduction: Moderate-to-severe plaque psoriasis is a chronic disease impacting quality of life (QoL). This network meta-analysis (NMA) compared efficacy and safety of all biologics approved for the treatment of moderate-to-severe plaque psoriasis to better inform providers on mid-term outcomes, with a focus on the interleukin-23 p19 inhibitor tildrakizumab.
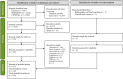
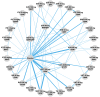
Methods: MEDLINE®, Embase, and CENTRAL were searched for randomized clinical trials (RCT) from inception through January 2024. RCTs comparing biologics against placebo or each other reporting Psoriasis Area and Severity Index (PASI), Physician Global Assessment (PGA) 0/1, or Dermatology Life Quality Index (DLQI) 0/1 responses and safety outcomes (adverse events [AEs] or serious AEs [SAEs]) were sought. Bayesian NMAs were performed at week 28 as the primary time point of interest. Analyses were also performed at weeks 12 and 16. Findings were expressed as risk ratios (RR; efficacy outcomes), risk differences (RD; safety outcomes), and numbers needed to treat (NNT) with 95% credible intervals.
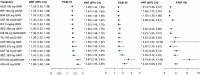
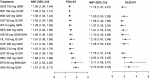
Results: Of 7418 publications screened, 187 describing 124 RCTs of 12 biologics were included in the systematic literature review, and 103 RCTs were included for NMA. All treatments demonstrated improved efficacy and QoL vs. placebo at week 28. Tildrakizumab efficacy at week 28 was comparable to risankizumab and guselkumab, respectively, for PASI 75 (RR 8.74 vs. 8.92 and 8.91), PASI 90 (RR 14.09 vs. 14.81 and 14.77), and PGA 0/1 (RR 9.34 vs. 10.29 and 10.23). No biologics exhibited an increased risk of SAEs vs. placebo; tildrakizumab exhibited no increased risk vs. placebo for AEs.
Conclusions: The investigated biologics demonstrated improved efficacy and QoL relative to placebo at week 28, with no increased risk of SAEs vs. placebo through week 16. At week 28, efficacy of tildrakizumab, risankizumab, and guselkumab was comparable. Limitations include lack of placebo comparators after week 12 or 16, which could affect results.
Keywords: Biologics; Network meta-analysis; Psoriasis; Systematic review; Tildrakizumab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Mark Lebwohl is an Editorial Board member of Dermatology and Therapy who was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. He is an employee of Mount Sinai; receives research funds from AbbVie, Arcutis, Avotres, Boehringer Ingelheim, Cara therapeutics, Clexio, Dermavant Sciences, Eli Lilly, Incyte, Inozyme, Janssen, Pfizer, Sanofi-Regeneron, and UCB; and is a consultant for Almirall, AltruBio Inc., Apogee, Arcutis, AstraZeneca, Atomwise, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Castle Biosciences, Celltrion, Corevitas, Dermavant Sciences, Dermsquared, Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Galderma, Genentech, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Sanofi-Regeneron, Seanergy, Strata, Takeda, Trevi, and Verrica. André Carvalho acted as a consultant and/or speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen Pharmaceuticals Inc., LEO Pharma, Novartis, Sun Pharma, and UCB. Akihiko Asahina has received honoraria and/or research grants from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Eli Lilly, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho Co., Ltd., Mitsubishi Tanabe Pharma, Novartis, Pfizer, Sun Pharma, Taiho Pharma, Torii Pharmaceutical Co., Ltd., and UCB. Jianzhong Zhang reports nothing to disclose. Mir Sohail Fazeli, Ellen Kasireddy, and Paul Serafini report employment with Evidinno Outcomes Research Inc. Thomas Ferro and Ranga Gogineni report employment with Sun Pharmaceutical Industries Inc. Diamant Thaçi is an advisor, speaker, or consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Galderma, Janssen, Kyowa Kirin, L'Oréal, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi/Genzyme, and UCB. Ethical Approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Figures
References
-
- National Psoriasis Foundation. Psoriasis statistics. https://www.psoriasis.org/psoriasis-statistics/. 2022. Accessed 23 July 2024.
-
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72. - PubMed
-
- American Academy of Dermatology. Psoriasis treatment: biologics. 2022. https://www.aad.org/public/diseases/psoriasis/treatment/medications/biol.... Accessed 18 Dec 2024.
-
- Blauvelt A, Noe MH. The best psoriasis medications emerge. JAMA Dermatol. 2024;160(1):99–100. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

