Self-administered versus lymphedema therapist-administered complex decongestive therapy protocol in breast cancer-related lymphedema: a non-inferiority randomized controlled trial with three-month follow-up
- PMID: 40329151
- PMCID: PMC12086114
- DOI: 10.1007/s10549-025-07709-3
Self-administered versus lymphedema therapist-administered complex decongestive therapy protocol in breast cancer-related lymphedema: a non-inferiority randomized controlled trial with three-month follow-up
Abstract
Purpose: The aim of this study was to demonstrate that a self-administered complex decongestive therapy (CDT) protocol is not inferior to certified lymphedema therapist (CLT)-administered CDT in the management of lymphedema and health-related outcomes in patients with breast cancer-related lymphedema (BCRL).
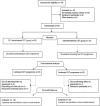
Methods: Fifty patients with BCRL were randomly assigned to two experimental groups: (1) a CLT-administered CDT group (n = 25) or a self-administered CDT group (n = 25). CDT was a multimodal approach in two experimental conditions consisting of patient education, manual lymph drainage, multi-layer bandaging, therapeutic exercises and skin/nail care. Lymphedema severity was assessed using circumference measurement, and BCRL-related symptoms were assessed using a numerical rating scale. The following measurement methods were used to assess health-related outcomes: universal goniometer for range of motion (ROMs), hand grip dynamometer for peripheral muscle strength, disabilities of the arm, shoulder and hand (DASH) questionnaire for disability level, International Physical Activity Questionnaire-Short Form (IPAQ-SF) for physical activity level, the checklist for individual strength (CIS) for fatigue and upper limb lymphedema quality of life questionnaire (ULL-27) for quality of life.
Results: Following CDT, there was a significant decrease in lymphedema severity and lymphedema-related symptoms in both groups (p < 0.001). There was no significant difference between the groups regarding the mean difference in health-related outcomes following CDT (post-treatment-baseline) (p < 0.05). Lymphedema severity and symptoms remained stable during the 3-month follow-up periods in the CLT-administered CDT group (p > 0.05). There was a decrease in the severity of lymphedema, stiffness, heaviness and fatigue in the self-administered CDT group at 3-month follow-up (p < 0.05), while pain and tingling remained unchanged (p > 0.05).
Conclusion: The present findings demonstrated self-administered CDT protocol is not inferior to CLT-administered CDT in the management of lymphedema and reduction of lymphedema-related disabilities.
Keywords: Breast cancer; Complex decongestive therapy; Health parameters; Self-care; Upper extremity lymphedema.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Ethical approval: Ethical approval was obtained from Dokuz Eylul University Non-Invasive Research Ethics Committee (decision number: 2023/34-09, date: 25.10.2023). Consent to participate: Informed consent was obtained from all participants and all procedures were performed in accordance with the Declaration of Helsinki.
Figures

Similar articles
-
Complex Decongestive Therapy Enhances Upper Limb Functions in Patients with Breast Cancer-Related Lymphedema.Lymphat Res Biol. 2018 Oct;16(5):446-452. doi: 10.1089/lrb.2017.0061. Epub 2018 Jan 22. Lymphat Res Biol. 2018. PMID: 29356592
-
Comparison of complete decongestive therapy and kinesiology taping for unilateral upper limb breast cancer-related lymphedema: A randomized controlled trial.Lymphology. 2021;54(1):41-51. Lymphology. 2021. PMID: 34506086 Clinical Trial.
-
Complex Decongestive Therapy in Breast Cancer-Related Lymphedema: Does Obesity Affect the Outcome Negatively?Lymphat Res Biol. 2019 Feb;17(1):45-50. doi: 10.1089/lrb.2017.0086. Epub 2018 Oct 3. Lymphat Res Biol. 2019. PMID: 30281384
-
Effectiveness of Lymphovenular Anastomosis and Complex Decongestive Therapy for the Treatment of Lymphedema in Patients with Breast Cancer: A Systematic Review.Lymphat Res Biol. 2024 Oct;22(5):232-240. doi: 10.1089/lrb.2024.0014. Epub 2024 Sep 24. Lymphat Res Biol. 2024. PMID: 39320336
-
Effectiveness of complete decongestive therapy for upper extremity breast cancer-related lymphedema: a review of systematic reviews.Med Oncol. 2024 Oct 23;41(11):297. doi: 10.1007/s12032-024-02421-6. Med Oncol. 2024. PMID: 39438358 Free PMC article.
References
-
- Hortobagyi GN (2003) The curability of breast cancer: present and future. Eur J Cancer Suppl 11:24–34. 10.1016/S1359-6349(03)00003-X
-
- Shih YC et al (2009) Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol 2712:2007–2014. 10.1200/jco.2008.18.3517 - PubMed
-
- (2020) The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 531: 3-19 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

