Safety, efficacy, and compliance of moderate-to-high dose eptinezumab and erenumab in chronic migraine patients with medication-overuse headache: an updated systematic review and meta-analysis
- PMID: 40329188
- PMCID: PMC12054139
- DOI: 10.1186/s10194-025-02047-7
Safety, efficacy, and compliance of moderate-to-high dose eptinezumab and erenumab in chronic migraine patients with medication-overuse headache: an updated systematic review and meta-analysis
Abstract
Background: The use of monoclonal antibodies targeting Calcitonin Gene-Related Peptide (CGRP) is an established treatment for chronic migraine (CM). However, its efficacy in CM patients with medication overuse headache (MOH) remains underexplored, and data on the safety and patient compliance of standard-to-high doses, especially Eptinezumab and Erenumab, over at least three months are limited.
Objective: This study aims to evaluate the efficacy and safety of anti-CGRP therapy (Eptinezumab and Erenumab) in CM and MOH patients. Specifically, it assesses changes in monthly migraine days (MMDs) after 12 weeks, risk of treatment-emergent adverse events (TEAEs) leading to discontinuation, serious TEAEs, common adverse effects, and MOH remission at 6 months.
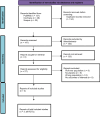
Methods: A systematic search of PubMed, Cochrane, and Scopus databases identified randomized controlled trials (RCTs) evaluating standard or high dose anti-CGRP therapy in CM patients strictly with MOH. Studies included were required to report a ≥ 50% reduction in MMDs after ≥ 12 weeks, serious TEAEs, TEAEs leading to discontinuation, common adverse events, and MOH remission at 6 months. Heterogeneity was assessed using I² statistics and a random-effects model.
Results: Three RCTs with 769 patients receiving standard-to-high dose anti-CGRP monoclonal antibodies (Eptinezumab and Erenumab) for ≥ 12 weeks were included. Anti-CGRP therapy significantly increased the likelihood of a ≥ 50% reduction in MMDs compared to placebo (OR: 2.43; 95% CI: 1.68-3.51; p < 0.00001). No substantial differences were found in TEAEs leading to discontinuation, nasopharyngitis, upper respiratory tract infections, or serious TEAEs between the anti-CGRP and placebo groups. The likelihood of MOH remission was approximately double in the anti-CGRP group (OR: 1.97; 95% CI: 1.40-2.78; p = 0.0001).
Conclusion: Standard-to-high dose anti-CGRP therapies (eptinezumab, erenumab) effectively reduce monthly migraine days and improve MOH remission rates with minimal adverse effects, showing good tolerability in CM patients with MOH.
Keywords: CM with MOH; Eptinezumab; Erenumab; MOH remission; Tolerability; ≥ 50% reduction in MMDs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: All authors report no relationships that could be construed as a conflict of interest. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Figures



Similar articles
-
Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication-overuse headache: Subgroup analysis of PROMISE-2.Headache. 2021 Jan;61(1):125-136. doi: 10.1111/head.14036. Epub 2020 Dec 13. Headache. 2021. PMID: 33314079 Clinical Trial.
-
The transition of medication overuse status by acute medication categories in episodic or chronic migraine patients to non-overuse status after receiving anti-CGRP monoclonal antibodies: a systematic review and meta-analysis of phase 3 randomized control trial.Neurol Sci. 2024 Sep;45(9):4451-4462. doi: 10.1007/s10072-024-07496-7. Epub 2024 Apr 2. Neurol Sci. 2024. PMID: 38564060
-
Persistent effectiveness of CGRP antibody therapy in migraine and comorbid medication overuse or medication overuse headache - a retrospective real-world analysis.J Headache Pain. 2024 Jul 4;25(1):109. doi: 10.1186/s10194-024-01813-3. J Headache Pain. 2024. PMID: 38965463 Free PMC article.
-
Efficacy and Safety of Erenumab in Adults With Medication Overuse Headache: Final Results From a Phase 4 Randomized Placebo-Controlled Study.Eur J Neurol. 2025 Aug;32(8):e70328. doi: 10.1111/ene.70328. Eur J Neurol. 2025. PMID: 40838472 Free PMC article. Clinical Trial.
-
Therapies targeting CGRP signaling for medication overuse headache.Curr Opin Neurol. 2022 Jun 1;35(3):353-359. doi: 10.1097/WCO.0000000000001061. Curr Opin Neurol. 2022. PMID: 35674079 Review.
References
-
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia (2018);38(1):1-211. 10.1177/0333102417738202 - PubMed
-
- Diener HC, Holle D, Solbach K et al (2016) Medication-overuse headache: risk factors, pathophysiology, and management. Nat Rev Neurol 12(10):575–583. 10.1038/nrneurol.2016.124 - PubMed
-
- Westergaard ML, Hansen EH, Glümer C et al (2014) Definitions of medication-overuse headache in population-based studies and their implications on prevalence estimates: A systematic review. Cephalalgia 34(6):409–425. 10.1177/0333102413512033 - PubMed
-
- Woldeamanuel YW, Cowan RP, Smith J et al (2017) Migraine affects 1 in 10 people worldwide featuring recent Rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci 372:307–315. 10.1016/j.jns.2016.11.071 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

