A pilot study on the effect of SARS-CoV-2 spike protein on IL-1β-mediated inflammation in peripheral blood immune cells from AIED patients
- PMID: 40329195
- PMCID: PMC12056982
- DOI: 10.1186/s10020-025-01227-0
A pilot study on the effect of SARS-CoV-2 spike protein on IL-1β-mediated inflammation in peripheral blood immune cells from AIED patients
Abstract
Background: Immune-mediated hearing loss (IMHL) patients (comprised of autoimmune inner ear disease (AIED) and sudden sensorineural hearing loss (SSNHL)) may be at higher risk for hearing loss following Coronavirus disease (COVID-19) infection and/or vaccination.
Methods: We compared inflammatory cytokine expression in response to SARS-CoV2 spike protein between two groups of patients with IMHL: IMHL patients that temporally demonstrated worsening SNHL following COVID vaccination or infection as compared to IMHL patients with worsening SNHL unrelated to COVID exposure: (IMHL-COVID ( +)) (n = 11) (IMHL-COVID (-)) (n = 10). In these two groups, we treated isolated PBMCs with increasing amounts of SARS-CoV-2 spike protein and compared responses to stimulation with positive and negative controls.
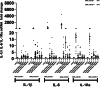
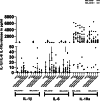
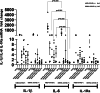
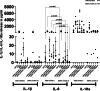
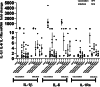
Results: Peripheral Blood Mononuclear Cells (PBMC) from IMHL-COVID ( +) patients had increased expression and release of both IL-1β and IL-6 in response to spike protein as compared to IMHL-COVID (-) patients. However, when the IMHL-COVID ( +) group was broken down into AIED patients compared to SSNHL, it became apparent that the greatest responses were from the AIED patients (p < 0.005 for IL-6 mRNA expression and p < 0.003 for IL-6 release when compared between any two similar groups using Wilcoxon Rank-Sum Test). When we broke down the COVID ( +) group to infection versus vaccination, the immune responses in the infection group (N = 3 AIED, 1 SSNHL) were stronger.
Conclusions: COVID-19 exposure with reported changes in hearing sensitivity in IMHL patients resulted in pro-inflammatory responses in response to spike protein. The inflammatory responses were greatest in AIED patients, and greater following infection rather than vaccination. Therefore, based on these studies, we would recommend AIED patients take additional precautions to avoid COVID exposure. Furthermore, we do recommend COVID vaccination during periods of hearing stability, as the immune responses are even more robust in response to infection in this vulnerable group.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The procedures using human biological samples were performed in accordance with institution regulations of Northwell Health System Institutional Review Board (IRB). All procedures were performed in accordance with ethical guidelines. Informed consent was provided by all enrolled patients prior to entering a subject into the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Colizza A, et al. Otolaryngology adverse events following COVID-19 vaccines. Eur Rev Med Pharmacol Sci. 2022;26:4113–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

