Clinical value of calibrated abdominal compression plus transthoracic echocardiography to predict fluid responsiveness in critically ill infants: a diagnostic accuracy study
- PMID: 40329198
- PMCID: PMC12057139
- DOI: 10.1186/s12887-025-05728-z
Clinical value of calibrated abdominal compression plus transthoracic echocardiography to predict fluid responsiveness in critically ill infants: a diagnostic accuracy study
Abstract
Background: Predicting fluid responsiveness is challenging in infants. It is however crucial to avoid unnecessary volume expansion, which can lead to fluid overload. We tested the hypothesis that the stroke volume changes induced by a calibrated abdominal compression (ΔSV-AC) could predict fluid responsiveness in infants without cardiac disease.
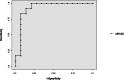
Methods: This prospective single center study of diagnostic test accuracy was conducted in a general pediatric intensive care unit (PICU). Children under the age of two with acute circulatory failure and requiring a 10 mL.kg-1 crystalloid volume expansion over 20 min, ventilated or not ventilated, were eligible. Stroke volume was measured by transthoracic echocardiography at baseline, during a gentle calibrated abdominal compression (22 mmHg for 30 s), and after volume expansion. The area under the receiver operating characteristic curve (AUROC) of ΔSV-AC was measured to predict fluid responsiveness, defined as a 15% stroke volume increase after volume expansion.
Results: Twenty-seven cases of volume expansion were analyzed, in 21 patients. Seventeen VE cases were administrated to spontaneously breathing children. Fluid responsiveness was observed in 12 cases. The AUROC of ΔSV-AC was 0.93 (95% confidence interval (95%CI) 0.82-1). The best threshold value for ΔSV-AC was 9.5%. At this threshold value, sensitivity was 92% (95%CI 62-100), specificity was 87% (95%CI 60-98), positive and negative predictive values were 85% (95%CI 60-95) and 93% (95%CI 66-99) respectively.
Conclusions: Echocardiographic assessment of stroke volume changes induced by a calibrated abdominal compression is a promising method to predict fluid responsiveness in infants without cardiac disease hospitalized in PICU.
Trial registration: ClinicalTrials.gov registration number NCT05919719, June 22, 2023, retrospectively registered, https://clinicaltrials.gov/study/NCT05919719 .
Keywords: Circulatory failure; Echocardiography; Fluid responsiveness; Goal-directed fluid management; Volume Expansion.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was carried out in accordance with the Good Clinical Practices protocol and Declaration of Helsinki principles. It was approved by our Institutional Review Board (Comité de Protection des Personnes Ouest IV, number 2021-A02876-35, approval date January 11 th 2022) and retrospectively registered on Clinicaltrials.gov (NCT05919719, June 22, 2023). Informed consent was obtained from all parents or legal guardians for minors, before or within 24 h after study procedures. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Van De Voorde P, Turner NM, Djakow J, De Lucas N, Martinez-Mejias A, Biarent D, et al. European Resuscitation Council Guidelines 2021: Paediatric Life Support. Resuscitation. 2021;161:327–87. - PubMed
-
- Gan H, Cannesson M, Chandler JR, Ansermino JM. Predicting Fluid Responsiveness in Children: A Systematic Review. Anesth Analg. 2013;117:1380–92. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

