Optimizing mesenchymal stem cell therapy: from isolation to GMP-compliant expansion for clinical application
- PMID: 40329219
- PMCID: PMC12054297
- DOI: 10.1186/s12860-025-00539-7
Optimizing mesenchymal stem cell therapy: from isolation to GMP-compliant expansion for clinical application
Abstract
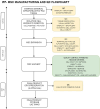
Background: Mesenchymal stem cells (MSCs) are promising for cell-based therapies targeting a wide range of diseases. However, challenges in translating MSC-based therapies to clinical applications necessitate standardized protocols following Good Manufacturing Practices (GMP) guidelines. This study aimed at developing GMP-complained protocols for FPMSCs isolation and manipulation, necessary for translational research, by (1) optimize culture of MSCs derived from an infrapatellar fat pad (FPMSC) condition through animal-free media comparison and (2) establish feasibility of MSC isolation, manufacturing and storage under GMP-compliance (GMP-FPMSC).
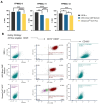
Methods: FPMSCs from three different patients were isolated following established protocols and the efficacy of two animal component-free media formulations in the culturing media were evaluated. The impact of different media formulations on cell proliferation, purity, and potency of MSCs was evaluated through doubling time, colony forming unit assay, and percentage of MSCs, respectively. Furthermore, the isolation and expansion of GMP-FPMSCs from four additional donors were optimized and characterized at each stage according to GMP requirements. Viability and sterility were checked using Trypan Blue and Bact/Alert, respectively, while purity and identity were confirmed using Endotoxin, Mycoplasma assays, and Flow Cytometry. The study also included stability assessments post-thaw and viability assessment to determine the shelf-life of the final GMP-FPMSC product. Statistical analyses were conducted using one-way ANOVA with Tukey's Multiple Comparisons.
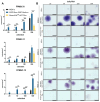
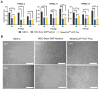
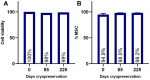
Results: The study demonstrated that FPMSCs exhibited enhanced proliferation rates when cultured in MSC-Brew GMP Medium compared to standard MSC media. Cells cultured in this media showed lower doubling times across passages, indicating increased proliferation. Additionally, higher colony formation in FPMSCs cultured in MSC-Brew GMP Medium were observed, supporting enhanced potency. Data from our GMP validation, including cells from 4 different donors, showed post-thaw GMP-FPMSC maintained stem cell marker expression and all the specifications required for product release, including > 95% viability (> 70% is required) and sterility, even after extended storage (up to 180 days), demonstrating the reproducibility and potential of GMP-FPMSCs for clinical use as well as the robustness of the isolation and storage protocols.
Conclusions: The study underscores the feasibility of FPMSCs for clinical uses under GMP conditions and emphasizes the importance of optimized culture protocols to improve cell proliferation and potency in MSC-based therapies.
Keywords: Good manufacturing practices; Infrapatellar fat pad-derived MSCs; Mesenchymal stem cells; Stem cell therapy; Translational research.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All methods were carried out in accordance with relevant guidelines and regulations. The experimental protocol was reviewed and approved by the Research Ethics Review Committee of Houston Methodist Hospital (Approval No. Pro00015718). Written informed consent was obtained from all participants prior to their inclusion in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Xeno-free protocol for GMP-compliant manufacturing of human fetal pancreas-derived mesenchymal stem cells.Stem Cell Res Ther. 2022 Jun 21;13(1):268. doi: 10.1186/s13287-022-02946-5. Stem Cell Res Ther. 2022. PMID: 35729640 Free PMC article.
-
Adipose tissue-derived mesenchymal stromal cells for clinical application: An efficient isolation approach.Curr Res Transl Med. 2019 Feb;67(1):20-27. doi: 10.1016/j.retram.2018.06.002. Epub 2018 Aug 10. Curr Res Transl Med. 2019. PMID: 30104160
-
GMP-manufactured density gradient media for optimized mesenchymal stromal/stem cell isolation and expansion.Cytotherapy. 2010 Jul;12(4):466-77. doi: 10.3109/14653241003649510. Cytotherapy. 2010. PMID: 20353309
-
Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications.Stem Cells Transl Med. 2014 May;3(5):643-52. doi: 10.5966/sctm.2013-0196. Epub 2014 Mar 28. Stem Cells Transl Med. 2014. PMID: 24682286 Free PMC article. Review.
-
Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells.Cells. 2021 Apr 13;10(4):886. doi: 10.3390/cells10040886. Cells. 2021. PMID: 33924517 Free PMC article. Review.
References
-
- Taraballi F, Pastò A, Bauza G, Varner C, Amadori A, Tasciotti E. Immunomodulatory potential of mesenchymal stem cell role in diseases and therapies: a bioengineering prospective. 2019;4:100017.
-
- Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J Orthop Res. 2013;31(9):1354–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

