Development and validation of a CT-based radiomics nomogram for predicting progression-free survival in patients with small cell lung cancer
- PMID: 40329257
- PMCID: PMC12057258
- DOI: 10.1186/s12880-025-01691-4
Development and validation of a CT-based radiomics nomogram for predicting progression-free survival in patients with small cell lung cancer
Abstract
Purpose: Small cell lung cancer (SCLC) is a highly aggressive form of lung cancer, representing about 15% of cases worldwide. Despite advances in imaging, such as low-dose CT, which have increased diagnostic rates, survival outcomes for SCLC patients have remained stagnant. Recent studies have only focused on radiomics, which extracts detailed quantitative features from imaging, with clinical risk factors to improve prognostic models. Therefore, this study aimed to develop a clinical-radiomics fusion nomogram based on computed tomography (CT) to estimate progression-free survival (PFS) in patients diagnosed with SCLC. By integrating radiomics features extracted from CT with clinical data, this model provides personalized prognostic assessment for clinicians. Its clinical utility lies in aiding treatment decision-making by offering more accurate prognostic evaluation, optimizing therapeutic strategies, and identifying high-risk patients at an early stage, ultimately improving overall survival and quality of life.
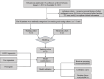
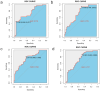
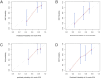
Methods: To develop the nomogram model, 95 patients diagnosed with pathologically confirmed SCLC between January 1, 2013, and December 31, 2023, were included in the study cohort. Participants were randomly divided into training and validation cohorts in a 7:3 ratio. Radiomics features associated with PFS were generated using the least absolute shrinkage and selection operator (LASSO) along with univariate and multivariate analyses. Additionally, in the training cohort, both univariate and multivariate analyses using Cox regression were conducted to identify the significant clinical risk factors influencing PFS. The predictive performance of the clinical and clinical-radiomics fusion nomogram were evaluated using the concordance index, calibration plots, and decision curve analysis (DCA).
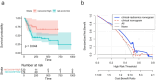
Results: Five radiomics features were selected and used to calculate the radiomics score (Rad-score). The radiomics features were significantly associated with PFS (hazard ratio: 0.5765, 95% confidence interval: 0.3641-0.9128, p < 0.05). Three clinical risk factors significantly associated with PFS were identified: neuron-specific enolase (NSE), carbohydrate antigen 125 levels (CA125), and treatment type, such as surgery. The clinical-radiomics fusion nomogram model (C-index:0.744) demonstrated superior performance compared to the clinical nomogram model (C-index: 0.718) in the training cohort. DCA indicated that the clinical-radiomics fusion nomogram outperformed the clinical nomogram in terms of clinical usefulness.
Conclusions: A CT-based clinical-radiomics fusion nomogram was developed to predict PFS in patients with SCLC, which is useful in providing individualized information.
Advances in knowledge: A clinical-radiomics fusion nomogram was constructed to estimate the probability of PFS based on clinical risk factors and the rad-score.
Keywords: Computed tomography; Nomogram; Progression-free survival; Radiomics; Small-cell lung cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Ethics Committee of Huadong Hospital, Affiliated with Fudan University, which waived the requirement for informed consent. All procedures were carried out in accordance with the relevant guidelines and regulations, and the study was conducted in accordance with the principles of the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
A CT-based radiomics nomogram for predicting the progression-free survival in small cell lung cancer: a multicenter cohort study.Radiol Med. 2023 Nov;128(11):1386-1397. doi: 10.1007/s11547-023-01702-w. Epub 2023 Aug 19. Radiol Med. 2023. PMID: 37597124
-
A CT-based radiomics model for predicting progression-free survival in patients with epithelial ovarian cancer.BMC Cancer. 2025 May 20;25(1):899. doi: 10.1186/s12885-025-14265-y. BMC Cancer. 2025. PMID: 40394512 Free PMC article.
-
Predicting overall survival and prophylactic cranial irradiation benefit in small cell lung cancer patients: a multicenter cohort study.BMC Cancer. 2024 Dec 6;24(1):1507. doi: 10.1186/s12885-024-13274-7. BMC Cancer. 2024. PMID: 39643886 Free PMC article.
-
Additional value of metabolic parameters to PET/CT-based radiomics nomogram in predicting lymphovascular invasion and outcome in lung adenocarcinoma.Eur J Nucl Med Mol Imaging. 2021 Jan;48(1):217-230. doi: 10.1007/s00259-020-04747-5. Epub 2020 May 25. Eur J Nucl Med Mol Imaging. 2021. PMID: 32451603
-
Sarculator: how to improve further prognostication of all sarcomas.Curr Opin Oncol. 2024 Jul 1;36(4):253-262. doi: 10.1097/CCO.0000000000001051. Epub 2024 Apr 30. Curr Opin Oncol. 2024. PMID: 38726834 Review.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. 10.3322/caac.21763. - PubMed
-
- Bernhardt EB, Jalal SI. Small cell lung Cancer. Cancer Treat Res. 2016;170:301–22. 10.1007/978-3-319-40389-2_14. - PubMed
-
- Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):725–37. 10.1038/nrc.2017.87. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

