FLT3LG modulates the infiltration of immune cells and enhances the efficacy of anti-PD-1 therapy in lung adenocarcinoma
- PMID: 40329265
- PMCID: PMC12057023
- DOI: 10.1186/s12885-025-14220-x
FLT3LG modulates the infiltration of immune cells and enhances the efficacy of anti-PD-1 therapy in lung adenocarcinoma
Abstract
Background: Immunotherapy, particularly anti-PD-1 therapy, has assumed a progressively significant position in the management of non-small cell lung cancer (NSCLC), especially in lung adenocarcinoma (LUAD). Nevertheless, a subset of patients exhibit resistance to anti-PD-1 therapy, and the exploration of biomarkers for evaluating the responsiveness to anti-PD-1 therapy necessitates further investigation. FLT3LG is regarded as being associated with tumor diagnosis and immunotherapy in a variety of tumor types, but its function in LUAD is uncertain.
Methods: Bioinformatics analysis was conducted to evaluate the clinical value, functional enrichment, genetic correlation, and immune infiltration of FLT3LG in LUAD. We then used a mouse model to detect immune cell infiltration and relevant protein expression by flow cytometry and immunohistochemistry under anti-PD-1 treatment after overexpression of FLT3LG. The serum FLT3LG expression in LUAD patients was detected via ELISA, and PD-L1 expression in tumor samples was detected by immunohistochemistry.
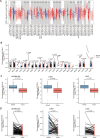
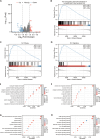
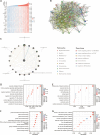
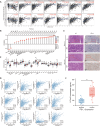
Results: In LUAD patients, a better prognosis is associated with elevated FLT3LG expression. Among the genes strongly associated with FLT3LG, the majority were involved in immune-related processes and were enriched predominantly in immune-related pathways. Moreover, high expression of FLT3LG was significantly positively correlated with increased infiltration of multiple immune cells, including T cells and natural killer (NK) cells, in lung adenocarcinomas, as well as the expression of several immune cell markers, such as CD4 and CD8a. In a mouse model, overexpression of FLT3LG in mice subjected to subcutaneous graft tumor elicited a pronounced immune response and could enhance the efficacy of anti-PD-1 therapy.
Conclusion: FLT3LG could be considered as a diagnostic and prognostic marker for LUAD and might play a role in enhancing the therapeutic response to immunotherapy in patients with LUAD.
Keywords: Biomarkers; Cancer immunotherapy; FLT3LG; Immune cell infiltration; Lung adenocarcinoma (LUAD).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments involving human samples and animals were authorized by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. All of the ethics guidelines pertaining to animal research were followed in terms of both animal care and experimentation. The ethics approval number is NO. XJTU1AF2021LSK-334. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Kim JW, Eder JP. Prospects for targeting PD-1 and PD-L1 in various tumor types. Oncol (Williston Park). 2014;28(Suppl 3):15–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

