Piezoelectric-immunomodulatory electrospun membrane for enhanced repair of refractory wounds
- PMID: 40329325
- PMCID: PMC12057002
- DOI: 10.1186/s12951-025-03393-z
Piezoelectric-immunomodulatory electrospun membrane for enhanced repair of refractory wounds
Abstract
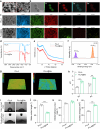
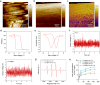
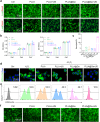
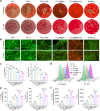
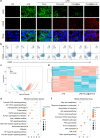
The microenvironment and healing process of diabetic wounds are highly complex, necessitating the development of wound dressings that combine excellent biocompatibility, superior antibacterial properties, and immune-regulating capabilities. However, achieving this goal remains a significant challenge. In this study, a multifunctional electrospun dressing (polylactic acid@Ga, PLLA@Ga) was designed and fabricated by integrating sonodynamic therapy with gallium-doped mesoporous bioactive glass (Ga-MBG). Compared to pure PLLA materials, PLLA@Ga exhibited remarkable antibacterial effects in vitro and demonstrated effective anti-infection properties in vivo. These effects are primarily attributed to the release of Ga ions, which competitively replace iron, thereby disrupting iron-dependent bacterial enzymes and ultimately leading to bacterial death. Additionally, in vitro experiments showed that PLLA@Ga could promote macrophage polarization from the M1 to M2 phenotype, effectively modulating the immune microenvironment of diabetic infected wounds. In vivo wound healing experiments further revealed that PLLA@Ga significantly enhanced collagen deposition and angiogenesis, accelerating the healing process of infected diabetic wounds. Thus, the multifunctional electrospun dressing developed in this study holds great potential as a promising candidate for the treatment of diabetic wounds.
Keywords: Antibacterial; Diabetic wounds; Electrospinning; Ga-MBG; Macrophage.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The procedures involving the animals and their care were conducted in conformity with national and international laws and policies. The animal experiments were approved by Changhai Hospital. Consent for publication: All authors agreed with the publisher to publish this work. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Chiral Polylactic acid membranes attenuate Hyperinflammation for accelerating wound healing.Int J Biol Macromol. 2025 Aug;319(Pt 3):145631. doi: 10.1016/j.ijbiomac.2025.145631. Epub 2025 Jun 27. Int J Biol Macromol. 2025. PMID: 40582682
-
Multifunctional coaxial gelatin/polylactic acid three-dimensional nanofibrous scaffolds for diabetic wounds.Int J Biol Macromol. 2025 May;306(Pt 3):141525. doi: 10.1016/j.ijbiomac.2025.141525. Epub 2025 Feb 26. Int J Biol Macromol. 2025. PMID: 40020849
-
Automatic in situ short-distance deposition of PLGA/PLLA composite nanofibrous membranes for personalized wound dressings.Nanoscale. 2024 May 2;16(17):8546-8562. doi: 10.1039/d3nr06376c. Nanoscale. 2024. PMID: 38596837
-
Glycopeptide-based multifunctional nanofibrous hydrogel that facilitates the healing of diabetic wounds infected with methicillin-resistant Staphylococcus aureus.Acta Biomater. 2024 Jun;181:161-175. doi: 10.1016/j.actbio.2024.04.035. Epub 2024 Apr 26. Acta Biomater. 2024. PMID: 38679405
-
An aligned porous electrospun fibrous membrane with controlled drug delivery - An efficient strategy to accelerate diabetic wound healing with improved angiogenesis.Acta Biomater. 2018 Apr 1;70:140-153. doi: 10.1016/j.actbio.2018.02.010. Epub 2018 Feb 15. Acta Biomater. 2018. PMID: 29454159
References
-
- Kuo S, Ye W, Wang D, McEwen LN, Villatoro Santos C, Herman WH. Cost-Effectiveness of the National Diabetes Prevention Program: A Real-World, 2-Year Prospective Study, Diabetes Care (2024). - PubMed
-
- Chu Z, Liu X, Zhao T, Jiang D, Zhao J, Dong X, Yeung KWK, Liu X, Liao Y, Ouyang L. Self-healing Ppy-hydrogel promotes diabetic skin wound healing through enhanced sterilization and macrophage orchestration triggered by NIR. Biomaterials. 2024;315:122964. - PubMed
-
- Zhang X, Ning F, Li Y, Lu J, He Y, Feng C, Dong CM. Pluripotent polysaccharide coordinated hydrogels remodel inflammation, neovascularization and reepithelization for efficient diabetic wound prohealing. J Control Release. 2024;377:37–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

