D- and L-Amino Acid Blood Concentrations Are Affected in Children With Duchenne Muscular Dystrophy
- PMID: 40329488
- PMCID: PMC12055753
- DOI: 10.1111/jcmm.70495
D- and L-Amino Acid Blood Concentrations Are Affected in Children With Duchenne Muscular Dystrophy
Abstract
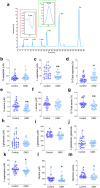
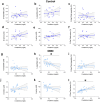
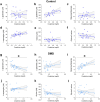
Duchenne muscular dystrophy (DMD) is an X-linked disease caused by the absence of functional dystrophin in the muscle cells. Recent untargeted metabolomics studies identified amino acid metabolism alterations as biochemical pathways potentially involved in DMD pathogenesis. Here, in a well-characterised cohort of DMD children and paediatric controls, we investigated by high-performance liquid chromatography (HPLC) the serum profile of a selected pool of amino acids in D- and L-configuration, including L-glutamate, L-glutamine, glycine, L-aspartate, D-aspartate, L-asparagine, L-serine, and D-serine. These amino acids are known to modulate neurotransmission and to play essential roles in energy and skeletal muscle metabolism. HPLC determinations highlighted a general amino acid deregulation in DMD compared to controls, including lower levels of L-aspartate, L-asparagine, D-serine, L-glutamine, and glycine and D-/Total serine ratio. In control subjects, we observed a significant positive correlation between L-glutamine and age, which lacked in affected children. Conversely, in DMD, we observed (i) a negative correlation of L-glutamate and L-aspartate with serum creatinine and creatine kinase levels; (ii) a direct correlation of serum L-glutamine/L-glutamate ratio with the fat-free mass index (as determined by dual energy X-ray absorptiometry) and with specific motor function scores (North Star Ambulatory Assessment); and (iii) no correlations between glucocorticoid treatment or cognitive function and the serum amino acid profile. Our study highlights significant correlations between serum L-glutamate levels, L-glutamine/L-glutamate ratio, and the multidimensional measures of muscle wasting and motor impairment, suggesting that peripheral glutamine-glutamate metabolism can be a suitable biomarker of disease severity and progression in DMD patients.
Keywords: Duchenne muscular dystrophy; D‐aspartate; D‐serine; amino acids; biomarker; glutamate; motor dysfunction; muscle wasting; serum.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

