Metformin use in women with polycystic ovary syndrome (PCOS): Opportunities, benefits, and clinical challenges
- PMID: 40329601
- PMCID: PMC12094230
- DOI: 10.1111/dom.16422
Metformin use in women with polycystic ovary syndrome (PCOS): Opportunities, benefits, and clinical challenges
Abstract
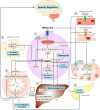
Metformin, a synthetic biguanide, is widely used to manage type 2 diabetes, and is commonly prescribed in polycystic ovary syndrome (PCOS) to address insulin resistance and associated metabolic and reproductive disturbances. PCOS is characterised by hormonal imbalances such as hyperandrogenism and anovulation, metabolic abnormalities including insulin resistance and increased cardiometabolic risk, and higher rates of pregnancy complications. However, the role of metformin in the multifaceted nature of PCOS remains debated. This review synthesises the mechanisms of action of metformin and its effects on metabolic, hormonal, reproductive, and pregnancy-related outcomes in PCOS. In non-pregnant women, metformin improves insulin resistance, menstrual regularity, and androgen levels, particularly in those with obesity or insulin resistance, and may enhance fertility when combined with other treatments. However, it is not effective as a first-line therapy for weight loss, ovulation induction, or treatment of clinical hyperandrogenic features, including hirsutism or acne. In pregnancy, metformin may reduce early pregnancy loss, miscarriage, and preterm birth, though findings for gestational diabetes and preeclampsia are inconsistent. Evidence is limited by study heterogeneity, varying diagnostic criteria, and the use of aggregate data in meta-analyses, all of which make interpretation challenging. Future research should prioritise well-powered clinical trials, individual patient data meta-analyses, and longer-term follow-up studies, particularly in pregnancy, to better define the populations most likely to benefit from metformin use across the PCOS spectrum. PLAIN LANGUAGE SUMMARY: Polycystic ovary syndrome (PCOS) is a common condition that affects up to 1 in 10 women of reproductive age. It is characterised by irregular or absent periods, signs of elevated male hormones (high androgens or excess hair growth), and/or polycystic ovaries seen on ultrasound. These features can lead to fertility problems, acne, psychological distress, and an increased risk of various disorders such as depression, type 2 diabetes and heart disease. Many women with PCOS also experience challenges during pregnancy, including a higher risk of miscarriage, preterm birth, and gestational diabetes. Metformin is a medication most often used to manage diabetes. In women with PCOS, it can help improve how the body responds to insulin, which may also reduce male hormone levels, improve menstrual cycles, and support fertility. This review examines the role of metformin in treating PCOS-both before and during pregnancy-by summarising key findings from the available evidence. In women who are not pregnant, metformin can help improve insulin resistance, hormone levels, and menstrual regularity, particularly among those who are overweight or have signs of insulin resistance. However, metformin alone is not a first-choice treatment for weight loss, ovulation problems, or symptoms such as acne and unwanted hair growth. When combined with other treatments, such as hormone therapy or fertility medications, it may offer additional benefits. During pregnancy, metformin is considered safe for use in women with PCOS and may lower the risk of early pregnancy loss and preterm birth. However, its effects on preventing gestational diabetes or high blood pressure are less clear, with mixed results across studies. Some research suggests that babies exposed to metformin in the womb may have slightly larger head sizes or a higher risk of being overweight in early childhood, but the long-term health effects remain unknown. Overall, metformin can be a helpful part of treatment for some women with PCOS, especially those with insulin resistance or certain pregnancy risks. Still, it is not a one-size-fits-all solution. More high-quality research is needed to better understand which women benefit most and to assess any long-term effects on children exposed to metformin during pregnancy.
Keywords: efficacy; infertility; metformin; polycystic ovary syndrome; pregnancy; reproductive health; review; safety.
© 2025 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Mousa A, Clef L, Costello M, Teede H. Technical Report for the Australian Adaptation of the ESHRE Evidence‐based guideline for unexplained infertility 2024. 2024.
-
- Teede HJ, Tay CT, Laven JJ, et al. Recommendations from the 2023 international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol. 2023;189(2):G43‐G64. - PubMed
-
- Joham AE, Norman RJ, Stener‐Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668‐680. - PubMed
-
- Salari N, Nankali A, Ghanbari A, et al. Global prevalence of polycystic ovary syndrome in women worldwide: a comprehensive systematic review and meta‐analysis. Arch Gynecol Obstet. 2024;310(3):1303‐1314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

