Evaluation of a score for identifying hospital stays that trigger a pharmacist intervention: integration into a clinical decision support system
- PMID: 40330112
- PMCID: PMC12051848
- DOI: 10.1093/jamiaopen/ooaf030
Evaluation of a score for identifying hospital stays that trigger a pharmacist intervention: integration into a clinical decision support system
Abstract
Objectives: The objective of the study was to determine, after medication review, the patient risk score threshold that would distinguish between stays with prescriptions triggering pharmacist intervention (PI) and stays with prescriptions not triggering PI.
Materials and methods: The study was retrospective and observational, conducted in the clinical pharmacy team. The patient risk score was adapted from a Canadian score and was integrated in the clinical decision support system (CDSS). For each hospital stay, the score was calculated at the beginning of hospitalization and we retrospectively showed if a medication review and a PI were conducted. Then, the optimal patient risk score threshold was determined to help pharmacist in optimizing medication review.
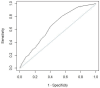
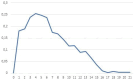
Results: During the study, 973 (56.7%) medication reviews were performed and 248 (25.5%) led to a PI. After analyzing sensitivity, specificity, and positive predictive value of different thresholds, the threshold of 4 was deemed discriminating to identify hospital stays likely to lead to a PI following a medication review. At this threshold, 600 hospital stays would have been detected (33.3% of the latter led to a PI), and 5.0% of stays with a medication review would not have been detected even though they were hospital stays that had triggered a PI.
Discussion and conclusion: Integration of a patient risk score in a CDSS can help clinical pharmacist to target hospital stays likely to trigger a PI. However, an optimal threshold is difficult to determine. Constructing and using a score in practice should be organized with the local clinical pharmacy team, in order to understand the tool's limitations and maximize its use in detecting at-risk drug prescriptions.
Keywords: clinical decision support system; clinical pharmacist; pharmacist intervention; scoring.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
Similar articles
-
Assessment of a hybrid decision support system using machine learning with artificial intelligence to safely rule out prescriptions from medication review in daily practice.Int J Clin Pharm. 2022 Apr;44(2):459-465. doi: 10.1007/s11096-021-01366-4. Epub 2022 Jan 3. Int J Clin Pharm. 2022. PMID: 34978662 Clinical Trial.
-
Detection of Drug-Related Problems through a Clinical Decision Support System Used by a Clinical Pharmacy Team.Healthcare (Basel). 2023 Mar 11;11(6):827. doi: 10.3390/healthcare11060827. Healthcare (Basel). 2023. PMID: 36981484 Free PMC article.
-
Identification of drug-related problems by a clinical pharmacist in addition to computerized alerts.Int J Clin Pharm. 2013 Oct;35(5):753-62. doi: 10.1007/s11096-013-9798-4. Epub 2013 May 29. Int J Clin Pharm. 2013. PMID: 23715760
-
Using Computer Alert Systems in the Emergency Room to Screen for Child Abuse [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Aug. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Aug. PMID: 38598650 Free Books & Documents. Review.
-
Risk Factors Associated with the Requirement for Pharmaceutical Intervention in the Hospital Setting: A Systematic Review of the Literature.Drugs Real World Outcomes. 2016 Sep;3(3):241-263. doi: 10.1007/s40801-016-0083-4. Drugs Real World Outcomes. 2016. PMID: 27747829 Free PMC article. Review.
References
-
- Commission staff working document—accompanying document to the :#Proposal for a Regulation of the European Parliament and of the Council amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No. 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency and the : #Proposal for a Directive of the European Parliament and of the Council amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use #Summary of the impact assessment {COM(2008) 664 final} {COM(2008) 665 final} {SEC(2008) 2670}. 2008.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous