Acoustofluidics-Based Intracellular Nanoparticle Delivery
- PMID: 40330125
- PMCID: PMC12054690
- DOI: 10.1016/j.eng.2024.11.030
Acoustofluidics-Based Intracellular Nanoparticle Delivery
Abstract
Controlled intracellular delivery of biomolecular cargo is critical for developing targeted therapeutics and cell reprogramming. Conventional delivery approaches (e.g., endocytosis of nano-vectors, microinjection, and electroporation) usually require time-consuming uptake processes, labor-intensive operations, and/or costly specialized equipment. Here, we present an acoustofluidics-based intracellular delivery approach capable of effectively delivering various functional nanomaterials to multiple cell types (e.g., adherent and suspension cancer cells). By tuning the standing acoustic waves in a glass capillary, our approach can push cells in flow to the capillary wall and enhance membrane permeability by increasing membrane stress to deform cells via acoustic radiation forces. Moreover, by coating the capillary with cargo-encapsulated nanoparticles, our approach can achieve controllable cell-nanoparticle contact and facilitate nanomaterial delivery beyond Brownian movement. Based on these mechanisms, we have successfully delivered nanoparticles loaded with small molecules or protein-based cargo to U937 and HeLa cells. Our results demonstrate enhanced delivery efficiency compared to attempts made without the use of acoustofluidics. Moreover, compared to conventional sonoporation methods, our approach does not require special contrast agents with microbubbles. This acoustofluidics-based approach creates exciting opportunities to achieve controllable intracellular delivery of various biomolecular cargoes to diverse cell types for potential therapeutic applications and biophysical studies.
Keywords: Acoustofluidics; Metal-organic frameworks; Nanocarriers; Sonoporation.
Conflict of interest statement
Declaration of competing interest Jason N. Belling, Liv K. Heidenreich, Steven J. Jonas, and Paul S. Weiss have patents related to this work. Zhishang Li, Zhenhua Tian, Joseph T. Rich, Haodong Zhu, Zhehan Ma, Hunter Bachman, Liang Shen, Yaosi Liang, Xiaolin Qi, Yao Gong, Shujie Yang, Wenfen Zhang, Peiran Zhang, Yingchun Fu, Yibin Ying, Yanbin Li, and Tony J. Huang declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
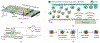




References
-
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet 2014;15(8):541–55. - PubMed
-
- van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, et al. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release 2006;112(2):149–55. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
