ACR-368, a CHK1/2 Kinase Inhibitor, in Patients With Relapsed or Refractory Desmoplastic Small Round Cell Tumor: Phase I/II Trial Results
- PMID: 40330140
- PMCID: PMC12052087
- DOI: 10.1200/OA-24-00095
ACR-368, a CHK1/2 Kinase Inhibitor, in Patients With Relapsed or Refractory Desmoplastic Small Round Cell Tumor: Phase I/II Trial Results
Abstract
Purpose: We hypothesized that ACR-368 (prexasertib) would be active in desmoplastic small round cell tumor (DSRCT) because of favorable responses in preclinical models.
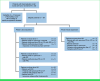
Methods: Preclinical work identified ACR-368 activity in DSRCT, and a phase I/II trial of ACR-368 and irinotecan in patients 12 months and older with relapsed/refractory DSRCT was conducted. The primary objectives were determination of recommended phase II dose (RP2D) and best overall response rate (ORR) at the RP2D in DSRCT, with ≥3 of 16 responses considered promising.
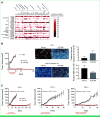
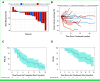
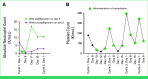
Results: Preclinical data confirmed ACR-368 as potentially therapeutic in DSRCT, and 19 patients were enrolled in a subsequent clinical trial. Treatment was well tolerated, and cytopenias were managed using growth factors. Fifteen of 19 patients, including five of six achieving PR, had previously received irinotecan. The estimated ORR at the RP2D was 23% (lower boundary one-sided 90% CI, 9%), exceeding the unpromising rate of 5%. In addition, three patients with DSRCT had a PR at doses other than the RP2D, bringing the ORR for all doses (n = 19) to 32% (90% CI, 15% to 53%). The median overall survival was 19 months (95% CI, 13 to 36).
Conclusion: The RP2D of ACR-368 with irinotecan by age group is ACR-368 105 or 150 mg/m2 once on day 1 (>21 years or ≤21 years, respectively) and irinotecan 15 mg/m2 once daily for 5 days in 21-day cycles for both groups. The study met its primary objective to consider ACR-368 and irinotecan promising in DSRCT and, to our knowledge, is the first incorporating a targeted therapy to achieve this magnitude of response.
© 2025 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to https://ascopubs.org/authors. Emily K. Slotkin Consulting or Advisory Role: Guidepoint Global, Inhibrx Research Funding: Lilly (Inst), Esperas Pharma Audrey Mauguen Patents, Royalties, Other Intellectual Property: Co-inventor of provisional patent Number 63/193,700, filed on May 27, 2021; conversion deadline: May 27, 2022; named “Soothsayer,” filed by Office of Technology Development, MSKCC Travel, Accommodations, Expenses: Northwestern Mutual Filemon S. Dela Cruz Research Funding: Eisai, Y-mAbs Therapeutics Paul A. Meyers Honoraria: France Foundation (I), Eastern Pulmonary Conference (I) Consulting or Advisory Role: Boehringer Ingelheim (I), Salarius Pharmaceuticals, US World Meds Speakers' Bureau: France Foundation (I), Genentech/Roche (I) Research Funding: Boehringer Ingelheim (I) Travel, Accommodations, Expenses: Takeda, InterMune (I) Leonard H. Wexler Consulting or Advisory Role: US WorldMeds Tara J. O'Donohue Employment: Amgen Stock and Other Ownership Interests: Amgen Research Funding: Bayer (Inst), Turning Point Therapeutics (Inst) Michael D. Kinnaman Employment: Regeneron Stock and Other Ownership Interests: Regeneron Ciara M. Kelly Employment: Daichii Sankyo (I) Stock and Other Ownership Interests: daichii sankyo (I) Consulting or Advisory Role: Kartos Therapeutics, SERVIER, Deciphera Research Funding: Amgen (Inst), Merck (Inst), Kartos Therapeutics (Inst), xencor (Inst), Servier (Inst), Regeneron (Inst), Curadev (Inst), IDRx (Inst), InhibRx (Inst) Travel, Accommodations, Expenses: Deciphera Sandra P. D'Angelo Honoraria: GlaxoSmithKline, Adaptimmune, Pfizer, Servier, Rain Therapeutics, Incyte, GI Innovation, AADi, Nektar Consulting or Advisory Role: Nektar, GlaxoSmithKline, Adaptimmune, Pfizer, Servier, Rain Therapeutics, Incyte, GI Innovation, AADi, Medendi Research Funding: EMD Serono, Amgen, Merck, Incyte, Nektar, Bristol Myers Squibb, Deciphera Travel, Accommodations, Expenses: Adaptimmune, EMD Serono, Nektar Other Relationship: GlaxoSmithKline, Nektar, Adaptimmune, Merck Mrinal M. Gounder Honoraria: Medscape, Guidepoint Global, Med Learning Group, Research to Practice, Great Debates and Updates, Gerson Lehrman Group, OncLive/MJH Life Sciences, MJH/PER Consulting or Advisory Role: Epizyme, Ayala Pharmaceuticals, Rain Therapeutics, AADi, Ikena Oncology, Kura Oncology Research Funding: Ayala Pharmaceuticals (Inst), AADi (Inst), Athenex (Inst), Boehringer Ingelheim (Inst), Foghorn Therapeutics (Inst), Ikena Oncology (Inst), GlaxoSmithKline (Inst), Rain Oncology (Inst), Regeneron (Inst), SpringWorks Therapeutics (Inst), SERVIER (Inst), Tango Therapeutics (Inst), Kymera (Inst), Erasca, Inc (Inst), Vivace Therapeutics (Inst) Patents, Royalties, Other Intellectual Property: UpToDate, GODDESS PRO Desmoid Tumor (Inst) Travel, Accommodations, Expenses: Epizyme Other Relationship: Desmoid Tumor Research Foundation Uncompensated Relationships: Foundation Medicine Open Payments Link: https://openpaymentsdata.cms.gov/physician/459583 Katherine Thornton Employment: Janssen Oncology Stock and Other Ownership Interests: Johnson & Johnson/Janssen Benjamin A. Nacev Travel, Accommodations, Expenses: Servier Ping Chi Stock and Other Ownership Interests: ORIC Pharmaceuticals (I) Consulting or Advisory Role: Deciphera, NewBay Pharma Research Funding: Deciphera (Inst), Pfizer (Inst), NewBay Pharma (Inst) Patents, Royalties, Other Intellectual Property: Royalties from ORIC (I) Travel, Accommodations, Expenses: NewBay Pharma Evan Rosenbaum Stock and Other Ownership Interests: Iovance Biotherapeutics, PMV Pharma Research Funding: Incyte, Arcus Biosciences, GlaxoSmithKline Mark Dickson Research Funding: Lilly (Inst), AADi (Inst), Sumitomo Dainippon Pharma Oncology (Inst) Romel Somwar Research Funding: Helsinn Healthcare (Inst), Elevation Oncology (Inst), Merus (Inst), Loxo (Inst), Loxo (Inst) Marc Ladanyi Stock and Other Ownership Interests: PAIGE.AI Consulting or Advisory Role: ADC Therapeutics, MSD, Bayer Health, Bayer Health, Merck, Gilead Sciences Research Funding: Merus NV (Inst), Elevation Oncology (Inst), Rain Therapeutics, ADC Therapeutics (Inst), Helsinn Therapeutics (Inst) Patents, Royalties, Other Intellectual Property: Royalties from a license agreement between MSK and Sophia Genetics Caroline Robb Employment: Remedy Plan, Inc Patents, Royalties, Other Intellectual Property: Remedy Plan, Inc patent (Inst) Travel, Accommodations, Expenses: Remedy Plan, Inc Neeta Pandit-Taskar Honoraria: Actinium Pharmaceuticals Consulting or Advisory Role: Actinium Pharmaceuticals, Telix Pharmaceuticals, Regeneron Speakers' Bureau: Telix Pharmaceuticals Research Funding: Imaginab (Inst), Regeneron (Inst), Bristol Myers Squibb (Inst), Janssen (Inst), Clarity Pharmaceuticals (Inst), Bayer Health (Inst), Fusion Pharmaceuticals (Inst), Y-mAbs Therapeutics Inc (Inst) Travel, Accommodations, Expenses: Bayer, Actinium Pharmaceuticals Damon R. Reed Consulting or Advisory Role: Eisai, SpringWorks Therapeutics Alex Kentsis Stock and Other Ownership Interests: Rgenta Therapeutics Consulting or Advisory Role: Novartis, Rgenta, Blueprint Medicines, Day One Therapeutics, Syndax Patents, Royalties, Other Intellectual Property: EMD Millipore Andrew L. Kung Leadership: Isabl Technologies Stock and Other Ownership Interests: Isabl Technologies Consulting or Advisory Role: DarwinHealth, Karyopharm Therapeutics Patents, Royalties, Other Intellectual Property: Licensing and royalty from bioluminescence imaging models, Licensing of technologies for cancer whole genome and transcriptome sequencing Julia Glade Bender Consulting or Advisory Role: Jazz Pharmaceuticals Research Funding: Eisai (Inst), Lilly (Inst), Loxo (Inst), Roche/Genentech (Inst), Bayer (Inst), Jazz Pharmaceuticals (Inst) Patents, Royalties, Other Intellectual Property: Patent on a T lymphoblastic lymphoma cell line, CUTLL1 Travel, Accommodations, Expenses: Amgen (Inst), Eisai (Inst) Uncompensated Relationships: SpringWorks Therapeutics, Bristol Myers Squibb, Merck, Eisai, Pfizer Open Payments Link: https://openpaymentsdata.cms.gov/physician/708514 William D. Tap Leadership: Certis Oncology Solutions, Atropos, AstraZeneca, Avacta Life Sciences Stock and Other Ownership Interests: Certis Oncology Solutions, Atropos Consulting or Advisory Role: Daiichi Sankyo, Deciphera, Servier, Boehringer Ingelheim, inhibrx, PharmaEssential, Aadi, Abbisko Therapeutics, Ikena Oncology, Ipsen, Bayer, C4 Therapeutics, Sonata, Avacta Life Sciences, IMGT, Curadev, Ratio Research Funding: Blueprint Medicines (Inst), BioAtla (Inst), Deciphera (Inst), Daiichi Sankyo (Inst), Theseus Pharmaceuticals (Inst), Avacta Life Sciences (Inst), Cogent Biosciences (Inst), C4 Therapeutics (Inst), servier (Inst), SpringWorks Therapeutics (Inst) Patents, Royalties, Other Intellectual Property: Companion Diagnostic for CDK4 inhibitors—14/854,329, Enigma and CDH18 as companion Diagnostics for CDK4 inhibition—SKI2016-021-03 No other potential conflicts of interest were reported.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
