Maintenance Capecitabine Plus Ramucirumab After First-Line Chemotherapy in Patients With Advanced Esophagogastric Adenocarcinoma: Results From the Randomized PLATFORM Study
- PMID: 40330143
- PMCID: PMC12053389
- DOI: 10.1200/OA-24-00073
Maintenance Capecitabine Plus Ramucirumab After First-Line Chemotherapy in Patients With Advanced Esophagogastric Adenocarcinoma: Results From the Randomized PLATFORM Study
Abstract
Purpose: PLATFORM is an adaptive phase II study assessing maintenance therapies in advanced esophagogastric adenocarcinoma (OGA). We evaluated the role of capecitabine plus a vascular endothelial growth factor receptor 2 inhibitor ramucirumab (cape-ram) in these patients.
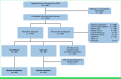
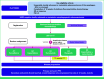
Methods: Human epidermal growth factor receptor 2 (HER2)-negative patients with advanced OGA with stable or responding disease after 18 weeks of induction platinum-based chemotherapy were randomly assigned 1:1 to surveillance or cape-ram. The primary end point was progression-free survival (PFS), and key secondary end points were overall survival (OS) and safety. Recruitment to the cape-ram arm closed prematurely because of industry support withdrawal. A one-sided log-rank test with a 2.5% significance level was considered significant.
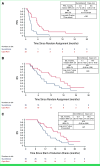
Results: Between April 2019 and November 2022, 25 surveillance and 22 cape-ram patients were contemporaneously randomly assigned. Median follow-up was 24.4 months. Compared with surveillance, cape-ram significantly prolonged PFS (hazard ratio [HR], 0.33 [95% CI, 0.17 to 0.63], P < .001; median PFS: 2.5 months with surveillance versus 5.5 months with cape-ram; 6-month PFS rate: 4% [95% CI, 0.3% to 17.0%] v 42.9% [95% CI, 21.9% to 62.3%], respectively) and OS (HR, 0.51 [95% CI, 0.26 to 1.00], P = .023; median OS: 7.1 months with surveillance v 14.4 months with cape-ram; median OS from start of induction chemotherapy was 12.1 months v 19.5 months, respectively). Of 10 cape-ram patients with measurable disease, 1 had an incremental partial response. Grade ≥3 adverse events (AEs) were seen in 32% surveillance and 57% cape-ram patients. Six cape-ram patients had grade 3 treatment-related AEs, and no new safety signals were identified.
Conclusion: Maintenance cape-ram after induction chemotherapy for patients with HER2-negative OGA significantly improved survival compared with surveillance. To our knowledge, this is the first randomized maintenance study demonstrating survival benefit and provides support for maintenance treatment.
© 2025 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to https://ascopubs.org/authors. Anderley Gordon Honoraria: Servier David Cunningham Stock and Other Ownership Interests: OVIBIO Consulting or Advisory Role: OVIBIO Research Funding: Bayer (Inst), 4SC (Inst), Clovis Oncology (Inst), Lilly (Inst), Leap Oncology (Inst), Roche (Inst) Caroline Fong Honoraria: Bristol Myers Squibb Michael Davidson Honoraria: Takeda, Roche China Travel, Accommodations, Expenses: Takeda Nicholas Maisey Consulting or Advisory Role: Servier/Pfizer Tom Waddell Stock and Other Ownership Interests: The Christie Clinic LLP Honoraria: Pfizer, Ipsen, Bristol Myers Squibb, EUSA Pharma Consulting or Advisory Role: Pfizer, Ipsen, Merck Sharp & Dohme, Eisai Europe, Merck Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Ipsen (Inst), Merck Sharp & Dohme (Inst), Roche (Inst), Eisai (Inst) Travel, Accommodations, Expenses: EUSA Pharma, Bristol Myers Squibb, Ipsen Carys Morgan Travel, Accommodations, Expenses: Novartis Alexander Bradshaw Honoraria: SERVIER Travel, Accommodations, Expenses: Servier Russell Petty Honoraria: Bristol Myers Squib, Servier Consulting or Advisory Role: Bristol-Myers Squib, Servier, BeiGene, Amgen, AstraZeneca, Astellas Pharma, Platinum Discovery Speakers' Bureau: Bristol Myers Squibb, Servier Research Funding: AstraZeneca, MSD Oncology (Inst), Merck Serono (Inst), Roche (Inst), Astellas Pharma (Inst), Moderna Therapeutics (Inst), Amgen (Inst), BioNTech SE (Inst), AbbVie (Inst), Astellas Pharma (Inst), Daiichi Sankyo/AstraZeneca (Inst), Platinum Discovery (Inst) Travel, Accommodations, Expenses: Bristol Myers Squibb, BeiGene, MSD Charlotte Fribbens Consulting or Advisory Role: Novartis Travel, Accommodations, Expenses: Novartis, Takeda Sheela Rao Honoraria: Bayer, Servier (I), Merck Serono, Incyte Consulting or Advisory Role: AstraZeneca, Bayer, Nordic Bioscience Speakers' Bureau: Takeda, Servier, Merck Serono, Incyte Travel, Accommodations, Expenses: Servier, MSD Oncology Naureen Starling Honoraria: Merck Serono, Servier, Pierre Fabre, MSD Oncology, Pierre Fabre, Seagen, GlaxoSmithKline, Merck, Pierre Fabre, MSD Oncology, BMS, AstraZeneca, Daiichi Sankyo, Natera, Astellas Pharma, Tempus Consulting or Advisory Role: MSD Oncology, GlaxoSmithKline, Gilead Sciences, Seagen, Janssen, Takeda, Moderna Therapeutics, Bristol Myers Squibb, AstraZeneca Research Funding: Gilead Sciences (Inst) Travel, Accommodations, Expenses: MSD Oncology, Guardant Health, Servier, GlaxoSmithKline, Takeda, Bristol Myers Squibb Uncompensated Relationships: Guardant Health Ian Chau Honoraria: Servier, Bristol Myers Squibb, Jazz Pharmaceuticals, Astellas Pharma, Daiichi Sankyo Consulting or Advisory Role: OncXerna Therapeutics, Astellas Pharma, GlaxoSmithKline, Seagen, BioNTech SE, Takeda, Elevation Oncology, BeiGene, Jazz Pharmaceuticals, Gilead Sciences, Roche/Genentech Research Funding: Janssen-Cilag (Inst), Lilly (Inst) Travel, Accommodations, Expenses: BMS, Servier, Takeda, Jazz Pharmaceuticals No other potential conflicts of interest were reported.
Figures
Similar articles
-
Randomized Phase II Study of Ramucirumab or Icrucumab in Combination with Capecitabine in Patients with Previously Treated Locally Advanced or Metastatic Breast Cancer.Oncologist. 2017 Mar;22(3):245-254. doi: 10.1634/theoncologist.2016-0265. Epub 2017 Feb 20. Oncologist. 2017. PMID: 28220020 Free PMC article. Clinical Trial.
-
Randomised phase II trial of trifluridine/tipiracil (FTD/TPI) plus ramucirumab (RAM) versus trifluridine/tipiracil for previously treated patients with advanced gastric or esophagogastric junction adenocarcinoma (RETRIEVE study, WJOG15822G).BMC Cancer. 2023 Aug 5;23(1):726. doi: 10.1186/s12885-023-11199-1. BMC Cancer. 2023. PMID: 37543568 Free PMC article. Clinical Trial.
-
Initial Report of Second-Line FOLFIRI in Combination with Ramucirumab in Advanced Gastroesophageal Adenocarcinomas: A Multi-Institutional Retrospective Analysis.Oncologist. 2019 Apr;24(4):475-482. doi: 10.1634/theoncologist.2018-0602. Epub 2018 Nov 23. Oncologist. 2019. PMID: 30470690 Free PMC article. Clinical Trial.
-
Clinical outcomes of ramucirumab plus docetaxel in the treatment of patients with non-small cell lung cancer after immunotherapy: a systematic literature review.Front Oncol. 2023 Sep 4;13:1247879. doi: 10.3389/fonc.2023.1247879. eCollection 2023. Front Oncol. 2023. PMID: 37731641 Free PMC article.
-
Salvage systemic therapy for advanced gastric and oesophago-gastric junction adenocarcinoma.Cochrane Database Syst Rev. 2020 Nov 19;11(11):CD012078. doi: 10.1002/14651858.CD012078.pub2. Cochrane Database Syst Rev. 2020. PMID: 33210731 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. - PubMed
-
- Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20:1996–2004. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
-
- Janjigian YY, Ajani JA, Moehler M, et al. LBA7 Nivolumab (NIVO) plus chemotherapy (chemo) or ipilimumab (IPI) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): CheckMate 649 study. Ann Oncol. 2021;32:S1329–S1330.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
